
The mercury-in-glass or mercury thermometer is a thermometer that uses the thermal expansion and contraction of liquid mercury to indicate the temperature.

The mercury-in-glass or mercury thermometer is a thermometer that uses the thermal expansion and contraction of liquid mercury to indicate the temperature.
A basic mercury thermometer is a precisely crafted piece of tube-shaped glass enveloping a mercury-filled reservoir connected to an extremely thin channel, called the capillary bore, that provides a chamber the mercury from the reservoir can expand into. The shorter, bulbous end of the tube containing the reservoir is called the bulb and the longer, narrower end with the bore is called the stem. Etched into the stem or on a carefully aligned plate next to it is a graduated temperature scale. Lower temperatures are near the bulb and higher temperatures near the top of the stem. The space above the mercury may be filled with nitrogen gas or it may be at less than atmospheric pressure, a partial vacuum. [1]
As the temperature of the surrounding environment changes, the mercury thermally expands and contracts, causing it to move out of, or into, the reservoir and, at the same time, rise or fall through the bore. Although changes to the mercury's volume are slight— about .018% for each degree Celsius [2] — the small volume of the bore compared to the bulb's volume visually amplifies the change. This design feature results in clearly visible movement of the mercury up or down the scale, enabling precise temperature readings.
In order to calibrate the thermometer, the bulb is made to reach thermal equilibrium with a temperature standard such as an ice/water mixture, and then with another standard such as water/vapour, and the tube is divided into regular intervals between the fixed points. In principle, thermometers made of different material (e.g., coloured alcohol thermometers) might be expected to give different intermediate readings due to different expansion properties; in practice the substances used are chosen to have reasonably linear expansion characteristics as a function of thermodynamic temperature, and so give similar results.

The earliest documented use of mercury in a thermometer stretches as far back to perhaps the 1620s when the Jesuit scholar, Athanasius Kircher, used quicksilver for his air thermometer, the precursor to in-glass thermometers. [3] : 23 Later, in the 1650s, failed experiments were run to determine if mercury might be a superior substitute for spirits in an enclosed glass thermometer. In 1659, the astronomer Ismael Boulliau abandoned using mercury when he determined that it was not as responsive to changes in temperature as spirits. [3] : 36–38
In 1713, Daniel Gabriel Fahrenheit began experimenting with mercury thermometers. By 1717, he was making them commercially. [3] : 79 The superiority of his mercury thermometers over alcohol-based thermometers made them very popular, leading to the widespread adoption of his Fahrenheit scale, the measurement system he developed and used for his thermometers. [4]
Anders Celsius, a Swedish scientist, devised the Celsius scale, which was described in his publication The origin of the Celsius temperature scale in 1742.
To define his scale Celsius used two fixed temperature points: the temperature of melting ice and the temperature of boiling water, both under atmospheric pressure of the standard atmosphere. This was not a new idea, since Isaac Newton was already working on something similar. The distinction of Celsius was to use the condition of melting and not that of freezing. The experiments for reaching a good calibration of his thermometer lasted for 2 winters. By performing the same experiment over and over again, he discovered that ice always melted at the same calibration mark on the thermometer. He found a similar fixed point in the calibration of boiling water to water vapour (when this is done to high precision, a variation will be seen with atmospheric pressure; Celsius noted this). At the moment that he removed the thermometer from the vapour, the mercury level climbed slightly. This was related to the rapid cooling (and contraction) of the glass.
When Celsius decided to use his own temperature scale, he originally defined his scale "upside-down", i.e. he chose to set the boiling point of pure water at 0 °C (212 °F) and the freezing point at 100 °C (32 °F). [5] One year later, Frenchman Jean-Pierre Christin proposed to invert the scale with the freezing point at 0 °C (32 °F) and the boiling point at 100 °C (212 °F). [6] He named it centigrade (100 steps).
Finally, Celsius proposed a method of calibrating a thermometer:
These points are adequate for approximate calibration, but both the freezing and boiling points of water vary with atmospheric pressure. Later thermometers that used a liquid other than mercury also gave slightly different temperature readings. In practice, these variations were very slight and remained close to the thermodynamic temperature, once the latter was discovered. These issues were explored experimentally with the gas thermometer. Until the discovery of true thermodynamic temperature, the mercury thermometer usually defined the temperature.
Modern thermometers are often calibrated using the triple point of water instead of the freezing point; the triple point occurs at 273.16 kelvins (K), 0.01 °C.


One special kind of mercury-in-glass thermometer, called a maximum thermometer, works by having a constriction in the neck close to the bulb. As the temperature rises, the mercury is pushed up through the constriction by the force of expansion. When the temperature falls, the column of mercury breaks at the constriction and cannot return to the bulb, thus remaining stationary in the tube. The observer can then read the maximum temperature over the set period of time. To reset the thermometer it must be swung sharply. This design is used in the traditional type of medical thermometer.
A maximum minimum thermometer, also known as Six's thermometer, is a thermometer which registers the maximum and minimum temperatures reached over a period of time, typically 24 hours. The original design contains mercury, but solely as a way to indicate the position of a column of alcohol whose expansion indicates the temperature; it is not a thermometer operated by the expansion of mercury; mercury-free versions are available.
Mercury thermometers cover a wide temperature range from −37 to 356 °C (−35 to 673 °F); the instrument's upper temperature range may be extended through the introduction of an inert gas such as nitrogen. This introduction of an inert gas increases the pressure on the liquid mercury and therefore its boiling point is increased, this in combination with replacing the Pyrex glass with fused quartz allows the upper temperature range to be extended to 800 °C (1,470 °F).
Mercury cannot be used below the temperature at which it becomes solid, −38.83 °C (−37.89 °F). If the thermometer contains nitrogen, the gas may flow down into the column when the mercury solidifies and be trapped there when the temperature rises, making the thermometer unusable until returned to the factory for reconditioning. To avoid this, some weather services require that all mercury-in-glass thermometers be brought indoors when the temperature falls to −37 °C (−35 °F).
To measure lower meteorological temperatures, a thermometer containing a mercury-thallium alloy which does not solidify until the temperature drops to −61.1 °C (−78.0 °F) may be used.
This section needs to be updated. The reason given is: 10+ year old information throughout.(May 2024) |
As of 2012 [update] , many mercury-in-glass thermometers are used in meteorology; however, they are becoming increasingly rare for other uses, as many countries banned them for medical use due to the toxicity of mercury. Some manufacturers use galinstan, a liquid alloy of gallium, indium, and tin, as a replacement for mercury.
The typical "fever thermometer" contains between 0.5 and 3 g (0.28 and 1.69 drachms ) of elemental mercury. [7] [8] Swallowing this amount of mercury would pose little danger but the inhaling of the vapour could lead to health problems. [9]

In February 2009, the Argentine Health Ministry instructed by resolution 139/09 that all health centres and hospitals should buy mercury-free thermometers and blood pressure meters and called on dentists, medical technicians, and environmental health specialists to start eliminating this toxin. [11] As of 2020 [update] , mercury thermometers were still on sale to the public at pharmacies.
There was a voluntary take-back action for thermometers containing mercury based on the Federal Waste Management Plan 2006, and carried out in close cooperation between the Austrian Chamber of Pharmacists (Österreichische Apothekerkammer), the Federal Ministry of Environment, a private waste disposer, a producer of electronic thermometers and a pharmaceutical distributor. The disposal company supplied each pharmacy (approximately 1,200) with a collection bin and covered the cost of disposal. The pharmaceutical distributor covered the logistical costs for the distribution of the thermometers. The pharmacies accepted a refund of only 0.50 Euro per thermometer for handling (which is far below their normal margin). The supplier provided the thermometers at a reduced price. The Federal Ministry supported each sold thermometer (covering about 30% of the direct costs) and advertised the project. During the collection period, consumers could bring in a mercury thermometer and buy an electronic thermometer for a subsidised price of 1 Euro. Between October 2007 and January 2008, about 465,000 electronic thermometers were sold and about one million mercury thermometers (together containing about 1 tonne of mercury) were collected. [12]
By the Philippines Department of Health’s Administrative Order 2008-0221, all mercury equipment from hospitals, including mercury-in-glass thermometers, was to be phased out in the Philippines by September 28, 2010. Even before the order was released, 50 hospitals had already banned mercury from their establishments. Among these fifty hospitals, the Philippine Heart Center was the first one to do so. San Juan de Dios Hospital, Philippine Children’s Medical Center, San Lazaro Hospital, Ospital ng Muntinlupa, Lung Center of the Philippines, the National Kidney and Transplant Institute, Manila Adventist Medical Center and Las Piñas Hospital also made steps to ban the toxic chemical. The country was the first one to make a step to ban mercury from its health care system in Southeast Asia and they used non-mercury digital thermometers instead. [13] [14]
Since European Union directive 2007/51/EC came into force on 3 April 2009, the UK Health Protection Agency (HPA) reported that mercury thermometers could no longer be sold to the general public. Shops holding stocks of unsold thermometers had to withdraw them from sale; mercury thermometers purchased before this date could be used without legal implications. The purpose of these restrictions is to protect the environment and public health by decreasing the amount of mercury waste released. [15] The HPA had, in 2007, released a guide to dealing with small spills of mercury. [16]
Despite the phasing-out of mercury thermometers in the United Kingdom, British media continues to refer to temperature measurements, especially for weather forecasts, as "the mercury". [17]
In the United States, both the American Academy of Pediatrics [18] and the United States Environmental Protection Agency [19] recommend that alternative thermometers be used in the home. [18]

In physics, cryogenics is the production and behaviour of materials at very low temperatures.

Daniel Gabriel Fahrenheit FRS was a physicist, inventor, and scientific instrument maker, born in Poland to a family of German extraction. Fahrenheit invented thermometers accurate and consistent enough to allow the comparison of temperature measurements between different observers using different instruments. Fahrenheit is also credited with inventing mercury-in-glass thermometers more accurate and superior to spirit-filled thermometers at the time. The popularity of his thermometers led to the widespread adoption of his Fahrenheit scale attached to his instruments.

The Fahrenheit scale is a temperature scale based on one proposed in 1724 by the European physicist Daniel Gabriel Fahrenheit (1686–1736). It uses the degree Fahrenheit as the unit. Several accounts of how he originally defined his scale exist, but the original paper suggests the lower defining point, 0 °F, was established as the freezing temperature of a solution of brine made from a mixture of water, ice, and ammonium chloride. The other limit established was his best estimate of the average human body temperature, originally set at 90 °F, then 96 °F.
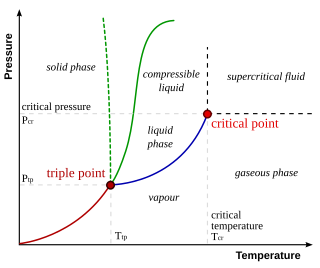
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases of that substance coexist in thermodynamic equilibrium. It is that temperature and pressure at which the sublimation, fusion, and vaporisation curves meet. For example, the triple point of mercury occurs at a temperature of −38.8 °C (−37.8 °F) and a pressure of 0.165 mPa.

A thermometer is a device that measures temperature or temperature gradient. A thermometer has two important elements: (1) a temperature sensor in which some change occurs with a change in temperature; and (2) some means of converting this change into a numerical value. Thermometers are widely used in technology and industry to monitor processes, in meteorology, in medicine, and in scientific research.

Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's thermodynamic tendency to evaporate. It relates to the balance of particles escaping from the liquid in equilibrium with those in a coexisting vapor phase. A substance with a high vapor pressure at normal temperatures is often referred to as volatile. The pressure exhibited by vapor present above a liquid surface is known as vapor pressure. As the temperature of a liquid increases, the attractive interactions between liquid molecules become less significant in comparison to the entropy of those molecules in the gas phase, increasing the vapor pressure. Thus, liquids with strong intermolecular interactions are likely to have smaller vapor pressures, with the reverse true for weaker interactions.

Boiling or ebullition is the rapid phase transition from liquid to gas or vapor; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere. Boiling and evaporation are the two main forms of liquid vapourization.
This is a timeline of temperature and pressure measurement technology or the history of temperature measurement and pressure measurement technology.
The following is a timeline of low-temperature technology and cryogenic technology. It also lists important milestones in thermometry, thermodynamics, statistical physics and calorimetry, that were crucial in development of low temperature systems.

The Réaumur scale, also known as the "octogesimal division", is a temperature scale for which the melting and boiling points of water are defined as 0 and 80 degrees respectively. The scale is named for René Antoine Ferchault de Réaumur, who first proposed a similar scale in 1730.

A hygrometer is an instrument which measures the humidity of air or some other gas: that is, how much water vapor it contains. Humidity measurement instruments usually rely on measurements of some other quantities such as temperature, pressure, mass and mechanical or electrical changes in a substance as moisture is absorbed. By calibration and calculation, these measured quantities can lead to a measurement of humidity. Modern electronic devices use the temperature of condensation, or they sense changes in electrical capacitance or resistance to measure humidity differences. A crude hygrometer was invented by Leonardo da Vinci in 1480. Major leaps came forward during the 1600s; Francesco Folli invented a more practical version of the device, while Robert Hooke improved a number of meteorological devices including the hygrometer. A more modern version was created by Swiss polymath Johann Heinrich Lambert in 1755. Later, in the year 1783, Swiss physicist and Geologist Horace Bénédict de Saussure invented the first hygrometer using human hair to measure humidity.

The Delisle scale is a temperature scale invented in 1732 by the French astronomer Joseph-Nicolas Delisle (1688–1768). Delisle was the author of Mémoires pour servir à l'histoire et aux progrès de l'Astronomie, de la Géographie et de la Physique (1738). The Delisle scale is notable as one of the few temperature scales that is inverted from the amount of thermal energy it measures; unlike most other temperature scales, higher measurements in degrees Delisle are colder, while lower measurements are warmer.

Psychrometrics is the field of engineering concerned with the physical and thermodynamic properties of gas-vapor mixtures.

The International Temperature Scale of 1990 (ITS-90) is an equipment calibration standard specified by the International Committee of Weights and Measures (CIPM) for making measurements on the Kelvin and Celsius temperature scales. It is an approximation of thermodynamic temperature that facilitates the comparability and compatibility of temperature measurements internationally. It defines fourteen calibration points ranging from 0.65 K to 1357.77 K and is subdivided into multiple temperature ranges which overlap in some instances. ITS-90 is the most recent of a series of International Temperature Scales adopted by the CIPM since 1927. Adopted at the 1989 General Conference on Weights and Measures, it supersedes the International Practical Temperature Scale of 1968 and the 1976 "Provisional 0.5 K to 30 K Temperature Scale". The CCT has also published several online guidebooks to aid realisations of the ITS-90. The lowest temperature covered by the ITS-90 is 0.65 K. In 2000, the temperature scale was extended further, to 0.9 mK, by the adoption of a supplemental scale, known as the Provisional Low Temperature Scale of 2000 (PLTS-2000).

Six's maximum and minimum thermometer is a registering thermometer that can record the maximum and minimum temperatures reached over a period of time, for example 24 hours. It is used to record the extremes of temperature at a location, for instance in meteorology and horticulture. It was invented by the British scientist James Six, in 1780; the same basic design remains in use.
A medical thermometer or clinical thermometer is a device used for measuring the body temperature of a human or other animal. The tip of the thermometer is inserted into the mouth under the tongue, under the armpit, into the rectum via the anus, into the ear, or on the forehead.

The alcohol thermometer or spirit thermometer has a similar construction and theory of operation as a mercury-in-glass thermometer. However, the thermometric fluid of an alcohol thermometer is less toxic and evaporates quickly making it a safer alternative to mercury thermometers. The ethanol version is the most widely used due to the low cost and relatively low hazard posed by the liquid in case of breakage.

The degree Celsius is the unit of temperature on the Celsius temperature scale, one of two temperature scales used in the International System of Units (SI), the other being the closely related Kelvin scale. The degree Celsius can refer to a specific point on the Celsius temperature scale or to a difference or range between two temperatures. It is named after the Swedish astronomer Anders Celsius (1701–1744), who proposed the first version of it in 1742. The unit was called centigrade in several languages for many years. In 1948, the International Committee for Weights and Measures renamed it to honor Celsius and also to remove confusion with the term for one hundredth of a gradian in some languages. Most countries use this scale.
Scale of temperature is a methodology of calibrating the physical quantity temperature in metrology. Empirical scales measure temperature in relation to convenient and stable parameters or reference points, such as the freezing and boiling point of water. Absolute temperature is based on thermodynamic principles: using the lowest possible temperature as the zero point, and selecting a convenient incremental unit.
A cryometer is a thermometer used to measure very low temperatures of objects. Ethanol-filled thermometers are used in preference to mercury for meteorological measurements and can measure temperatures in the range of -38 to 70 °C. Their main physical limitation is the freezing and boiling point of the liquid used.
1743 Jean-Pierre Christin inverts the fixed points on Celsius' scale, to produce the scale used today.
* oral/rectal/baby thermometers, containing about 0.61 grams of mercury; and
* basal temperature thermometers, containing about 2.25 grams of mercury.