Related Research Articles

Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.

Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events. Most commonly, protein phosphorylation is catalyzed by protein kinases, ultimately resulting in a cellular response. Proteins responsible for detecting stimuli are generally termed receptors, although in some cases the term sensor is used. The changes elicited by ligand binding in a receptor give rise to a biochemical cascade, which is a chain of biochemical events known as a signaling pathway.

The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components: microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth or disassembly depending on the cell's requirements.

The sodium–potassium pump is an enzyme found in the membrane of all animal cells. It performs several functions in cell physiology.

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins.
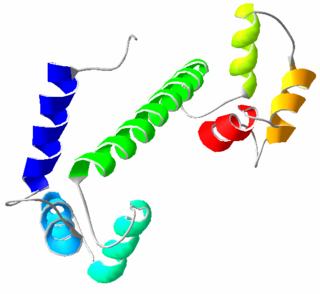
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases.

Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms' cells. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction of all muscle cell types, and in fertilization. Many enzymes require calcium ions as a cofactor, including several of the coagulation factors. Extracellular calcium is also important for maintaining the potential difference across excitable cell membranes, as well as proper bone formation.

A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst. Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound.
Bioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well as artificially introduced metals, including those that are non-essential, in medicine and toxicology. Many biological processes such as respiration depend upon molecules that fall within the realm of inorganic chemistry. The discipline also includes the study of inorganic models or mimics that imitate the behaviour of metalloproteins.
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. Second messengers trigger physiological changes at cellular level such as proliferation, differentiation, migration, survival, apoptosis and depolarization.
In biology, cell signaling is the process by which a cell interacts with itself, other cells, and the environment. Cell signaling is a fundamental property of all cellular life in prokaryotes and eukaryotes.
QPNC-PAGE, or QuantitativePreparativeNativeContinuousPolyacrylamideGel Electrophoresis, is a bioanalytical, one-dimensional, high-resolution and high-precision electrophoresis technique applied in biochemistry and bioinorganic chemistry to separate proteins quantitatively by isoelectric point and by continuous elution from a gel column.

Iron is an important biological element. It is used in both the ubiquitous iron-sulfur proteins and in vertebrates it is used in hemoglobin which is essential for blood and oxygen transport.

Phospholipase C (PLC) is a class of membrane-associated enzymes that cleave phospholipids just before the phosphate group (see figure). It is most commonly taken to be synonymous with the human forms of this enzyme, which play an important role in eukaryotic cell physiology, in particular signal transduction pathways. Phospholipase C's role in signal transduction is its cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which serve as second messengers. Activators of each PLC vary, but typically include heterotrimeric G protein subunits, protein tyrosine kinases, small G proteins, Ca2+, and phospholipids.
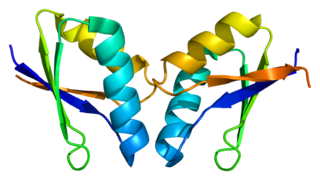
ATOX1 is a copper metallochaperone protein that is encoded by the ATOX1 gene in humans. In mammals, ATOX1 plays a key role in copper homeostasis as it delivers copper from the cytosol to transporters ATP7A and ATP7B. Homologous proteins are found in a wide variety of eukaryotes, including Saccharomyces cerevisiae as ATX1, and all contain a conserved metal binding domain.

Cell surface receptors are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving extracellular molecules. They are specialized integral membrane proteins that allow communication between the cell and the extracellular space. The extracellular molecules may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules, or nutrients; they react with the receptor to induce changes in the metabolism and activity of a cell. In the process of signal transduction, ligand binding affects a cascading chemical change through the cell membrane.
Amy C. Rosenzweig is a professor of Chemistry and Molecular Biosciences at Northwestern University. She was born in 1967 in Pittsburgh, Pennsylvania. Her current research interests include structural biology and bioinorganic chemistry, metal uptake and transport, oxygen activation by metalloenzymes, and characterization of membrane protein. For her work, she has been recognized by a number of national and international awards, including the MacArthur "Genius" Award in 2003.
Cation diffusion facilitators (CDFs) are transmembrane proteins that provide tolerance of cells to divalent metal ions, such as cadmium, zinc, and cobalt. These proteins are considered to be efflux pumps that remove these divalent metal ions from cells. However, some members of the CDF superfamily are implicated in ion uptake. All members of the CDF family possess six putative transmembrane spanners with strongest conservation in the four N-terminal spanners. The Cation Diffusion Facilitator (CDF) Superfamily includes the following families:
The nik operon is an operon required for uptake of nickel ions into the cell. It is present in many bacteria, but has been extensively studied in Helicobacter pylori. Nickel is an essential nutrient for many microorganisms, where it participates in a variety of cellular processes. However, excessive levels of nickel ions in cell can be fatal to the cell. Nickel ion concentration in the cell is regulated through the nik operon.
Sabeeha Sabanali Merchant is a professor of plant biology at the University of California, Berkeley. She studies the photosynthetic metabolism and metalloenzymes In 2010 Merchant led the team that sequenced the Chlamydomonas genome. She was elected a member of the National Academy of Sciences in 2012.
References
- ↑ Rosenzweig, Amy C (2002-06-01). "Metallochaperones: Bind and Deliver". Chemistry & Biology. 9 (6): 673–677. doi: 10.1016/S1074-5521(02)00156-4 . ISSN 1074-5521. PMID 12079778.
- 1 2 Finney LA, O'Halloran TV (2003-05-09). "Transition metal speciation in the cell: insights from the chemistry of metal ion receptors". Science. 300 (5621): 931–6. Bibcode:2003Sci...300..931F. doi:10.1126/science.1085049. PMID 12738850. S2CID 14863354.
- 1 2 Sekhon, Bhupinder Singh (2010-05-01). "Metallochaperones - an Overview". Current Chemical Biology. 4 (2): 173–186. doi:10.2174/2212796811004020173 . Retrieved 2019-01-21.