In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

Butanone, also known as methyl ethyl ketone (MEK) or ethyl methyl ketone, is an organic compound with the formula CH3C(O)CH2CH3. This colorless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran.
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzene (hemimellitene). All three compounds have the formula C6H3(CH3)3, which is commonly abbreviated C6H3Me3. Mesitylene is a colorless liquid with sweet aromatic odor. It is a component of coal tar, which is its traditional source. It is a precursor to diverse fine chemicals. The mesityl group (Mes) is a substituent with the formula C6H2Me3 and is found in various other compounds.
Methyl ethyl ketone peroxide (MEKP) is an organic peroxide with the formula [(CH3)(C2H5)C(O2H)]2O2. MEKP is a colorless oily liquid. It is widely used in vulcanization (crosslinking) of polymers.
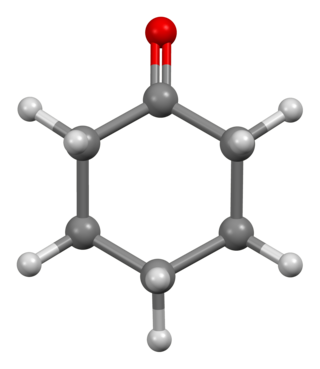
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of cyclohexanone assume a pale yellow color. Cyclohexanone is slightly soluble in water and miscible with common organic solvents. Billions of kilograms are produced annually, mainly as a precursor to nylon.

Acetone is an organic compound with the formula (CH3)2CO. It is the simplest and smallest ketone. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor.

Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA).

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.

Mesityl oxide is a α,β-unsaturated ketone with the formula CH3C(O)CH=C(CH3)2. This compound is a colorless, volatile liquid with a honey-like odor.

Aleksandr Pavlovich Dianin was a Russian Empire chemist from Saint Petersburg. He carried out studies on phenols and discovered a phenol derivative now known as bisphenol A and the accordingly named Dianin's compound. He was married to the adopted daughter of fellow chemist Alexander Borodin. In 1887, Dianin succeeded his father-in-law as chair of the Chemistry Department at the Imperial Medical-Surgical Academy in St. Petersburg.
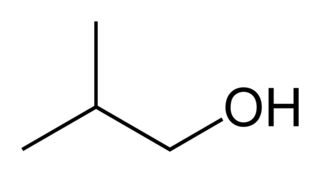
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as i-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either directly or as its esters. Its isomers are 1-butanol, 2-butanol, and tert-butanol, all of which are important industrially.

Methacrylic acid, abbreviated MAA, is an organic compound with the formula CH2=C(CH3)COOH. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA).

2-Ethoxyethanol, also known by the trademark Cellosolve or ethyl cellosolve, is a solvent used widely in commercial and industrial applications. It is a clear, colorless, nearly odorless liquid that is miscible with water, ethanol, diethyl ether, acetone, and ethyl acetate.

Methyl vinyl ketone (MVK, IUPAC name: butenone) is the organic compound with the formula CH3C(O)CH=CH2. It is a reactive compound classified as an enone, in fact the simplest example thereof. It is a colorless, flammable, highly toxic liquid with a pungent odor. It is soluble in water and polar organic solvents. It is a useful intermediate in the synthesis of other compounds.
Diisopropylamine is a secondary amine with the chemical formula (Me2CH)2NH (Me = methyl). Diisopropylamine is a colorless liquid with an ammonia-like odor. Its lithium derivative, lithium diisopropylamide, known as LDA is a widely used reagent.
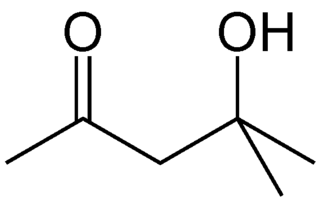
Diacetone alcohol is an organic compound with the formula CH3C(O)CH2C(OH)(CH3)2, sometimes called DAA. This colorless liquid is a common synthetic intermediate used for the preparation of other compounds, and is also used as a solvent.

Isophorone is an α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppermint-like odor, although commercial samples can appear yellowish. Used as a solvent and as a precursor to polymers, it is produced on a large scale industrially.
Acetone cyanohydrin (ACH) is an organic compound used in the production of methyl methacrylate, the monomer of the transparent plastic polymethyl methacrylate (PMMA), also known as acrylic. It liberates hydrogen cyanide easily, so it is used as a source of such. For this reason, this cyanohydrin is also highly toxic.

Pinacolborane is the borane with the formula (CH3)4C2O2BH. Often pinacolborane is abbreviated HBpin. It features a boron hydride functional group incorporated in a five-membered C2O2B ring. Like related boron alkoxides, pinacolborane is monomeric. It is a colorless liquid. It features a reactive B-H functional group.

α,β-Unsaturated carbonyl compounds are organic compounds with the general structure (O=CR)−Cα=Cβ-R. Such compounds include enones and enals, but also carboxylic acids and the corresponding esters and amides. In these compounds the carbonyl group is conjugated with an alkene. Unlike the case for carbonyls without a flanking alkene group, α,β-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the β-carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction.