
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams, analogous to “R” used for any organic substituent. “Ar” is not to be confused with the elemental symbol for argon.
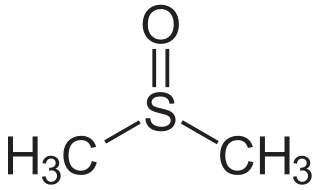
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula (CH3)2SO. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO has the unusual property that many individuals perceive a garlic-like taste in the mouth after DMSO makes contact with their skin.
Demeton-S-methyl is an organic compound with the molecular formula C6H15O3PS2. It was used as an organothiophosphate acaricide and organothiophosphate insecticide. It is flammable. With prolonged storage, Demeton-S-methyl becomes more toxic due to formation of a sulfonium derivative which has greater affinity to the human form of the acetylcholinesterase enzyme, and this may present a hazard in agricultural use.
The Robinson annulation is a chemical reaction used in organic chemistry for ring formation. It was discovered by Robert Robinson in 1935 as a method to create a six membered ring by forming three new carbon–carbon bonds. The method uses a ketone and a methyl vinyl ketone to form an α,β-unsaturated ketone in a cyclohexane ring by a Michael addition followed by an aldol condensation. This procedure is one of the key methods to form fused ring systems.
The Pummerer rearrangement is an organic reaction whereby an alkyl sulfoxide rearranges to an α-acyloxy–thioether (monothioacetal-ester) in the presence of acetic anhydride.
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.

In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.
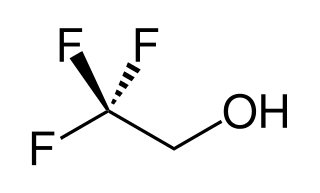
2,2,2-Trifluoroethanol is the organic compound with the formula CF3CH2OH. Also known as TFE or trifluoroethyl alcohol, this colourless, water-miscible liquid has a smell reminiscent of ethanol. Due to the electronegativity of the trifluoromethyl group, this alcohol exhibits a stronger acidic character compared to ethanol.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols. An exception is the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act. The list can be found as an appendix to 40 C.F.R. 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121.
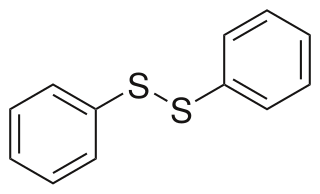
Diphenyl disulfide is the chemical compound with the formula (C6H5S)2. This colorless crystalline material is often abbreviated Ph2S2. It is one of the more commonly encountered organic disulfides in organic synthesis. Minor contamination by thiophenol is responsible for the disagreeable odour associated with this compound.
In organic chemistry, the Ei mechanism, also known as a thermal syn elimination or a pericyclic syn elimination, is a special type of elimination reaction in which two vicinal (adjacent) substituents on an alkane framework leave simultaneously via a cyclic transition state to form an alkene in a syn elimination. This type of elimination is unique because it is thermally activated and does not require additional reagents, unlike regular eliminations, which require an acid or base, or would in many cases involve charged intermediates. This reaction mechanism is often found in pyrolysis.

Dichloropane ((−)-2β-Carbomethoxy-3β-(3,4-dichlorophenyl)tropane, RTI-111, O-401) is a stimulant of the phenyltropane class that acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) with IC50 values of 3.13, 0.79 and 18 nM, respectively. In animal studies, dichloropane had a slower onset and longer duration of action compared to cocaine.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

Thiopropamine is a stimulant drug which is an analogue of amphetamine where the phenyl ring has been replaced by thiophene. It has similar stimulant effects to amphetamine but with around one third the potency. The N-methyl and thiophen-3-yl analogues are also known and are somewhat more potent, though still generally weaker than the corresponding amphetamines.

Leptophos (O-(4-bromo-2,5-dichlorophenyl) O-methyl phenylphosphonothioate) belongs to the organophosphates and at room temperature it is a stable white solid. It is also known as Phosvel, Abar and Vcs 506. Leptophos was primarily used as a pesticide and fungicide. for rice, cotton, fruit and vegetables until its use was discontinued in 1975 in USA, but still sold in South-Eastern Asia in 1981.

Ethiofencarb is a carbamate insecticide which is useful in controlling aphids on hard and soft fruits and some vegetables. It is not as dangerous as organophosphorous pesticides, but is considered highly toxic to humans in the UK, moderately toxic under US EPA classification, and highly toxic to aquatic life.
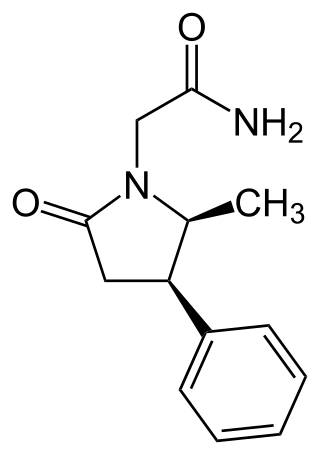
Methylphenylpiracetam is a derivative of piracetam and a positive allosteric modulator of the sigma-1 receptor. It differs from phenylpiracetam by having a methyl group.

Thioanisole is an organic compound with the formula CH3SC6H5. It is a colorless liquid that is soluble in organic solvents. It is the simplest alkyl–aryl thioether. The name indicates that this compound is the sulfur analogue—the thioether rather than the oxygen-centered ether—of anisole.
















