In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula CH3. In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond, it can be found on its own in any of three forms: methanide anion, methylium cation or methyl radical. The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed.
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and the biological sciences.

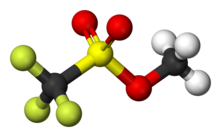
In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group R−S(=O)2−O−, where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non-oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates.
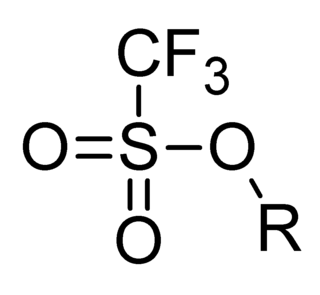
In organic chemistry, triflate, is a functional group with the formula R−OSO2CF3 and structure R−O−S(=O)2−CF3. The triflate group is often represented by −OTf, as opposed to −Tf, which is the triflyl group, R−SO2CF3. For example, n-butyl triflate can be written as CH3CH2CH2CH2OTf.
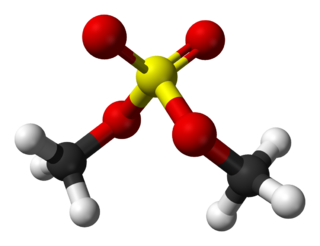
Dimethyl sulfate (DMS) is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as (CH3)2SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agent in organic synthesis.
![<span class="mw-page-title-main">Sulfonium</span> Cation of the form [SR3]+](https://upload.wikimedia.org/wikipedia/commons/thumb/6/62/%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png/320px-%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png)
In organic chemistry, a sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively-charged ion featuring three organic substituents attached to sulfur. These organosulfur compounds have the formula [SR3]+. Together with a negatively-charged counterion, they give sulfonium salts. They are typically colorless solids that are soluble in organic solvent.
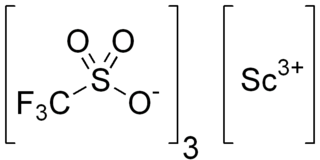
Scandium trifluoromethanesulfonate, commonly called scandium triflate, is a chemical compound with formula Sc(SO3CF3)3, a salt consisting of scandium cations Sc3+ and triflate SO
3CF−
3 anions.

Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for esterification. It is a hygroscopic, colorless, slightly viscous liquid and is soluble in polar solvents.
Pivalic acid is a carboxylic acid with a molecular formula of (CH3)3CCO2H. This colourless, odiferous organic compound is solid at room temperature. A common abbreviation for the pivalyl or pivaloyl group (t-BuC(O)) is Piv and for pivalic acid (t-BuC(O)OH) is PivOH.

Dimethyl carbonate (DMC) is an organic compound with the formula OC(OCH3)2. It is a colourless, flammable liquid. It is classified as a carbonate ester. This compound has found use as a methylating agent and more recently as a solvent that is exempt from the restrictions placed on most volatile organic compounds (VOCs) in the US. Dimethyl carbonate is often considered to be a green reagent.
Lanthanide triflates are triflate salts of the lanthanides. These salts have been investigated for application in organic synthesis as Lewis acid catalysts. These catalysts function similarly to aluminium chloride or ferric chloride, but they are water-tolerant (stable in water). Commonly written as Ln(OTf)3·(H2O)9 the nine waters are bound to the lanthanide, and the triflates are counteranions, so more accurately lanthanide triflate nonahydrate is written as [Ln(H2O)9](OTf)3.
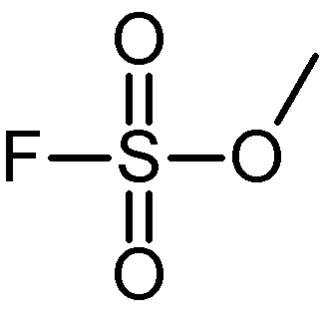
Methyl fluorosulfonate, also known as magic methyl, is the organic compound with the formula FSO2OCH3. It is a colorless liquid that is used as a strong methylating agent in organic synthesis. Because of its extreme toxicity, it has largely been replaced by the related reagent methyl trifluoromethanesulfonate.

Silver trifluoromethanesulfonate, or silver triflate is the triflate (CF3SO3−) salt of Ag+. It is a white or colorless solid that is soluble in water and some organic solvents including, benzene. It is a reagent used in the synthesis of organic and inorganic triflates.

In organic chemistry, the triflyl group is a functional group with the formula R−SO2CF3 and structure R−S(=O)2−CF3. The triflyl group is often represented by –Tf.
Living cationic polymerization is a living polymerization technique involving cationic propagating species. It enables the synthesis of very well defined polymers and of polymers with unusual architecture such as star polymers and block copolymers and living cationic polymerization is therefore as such of commercial and academic interest.

Trimethyloxonium tetrafluoroborate is the organic compound with the formula (CH3)3OBF4. This salt is a strong methylating agent, being a synthetic equivalent of CH+3. It is a white solid that rapidly degrades upon exposure to atmospheric moisture, although it is robust enough to be weighed quickly without inert atmosphere protection. Triethyloxonium tetrafluoroborate is a closely related compound.
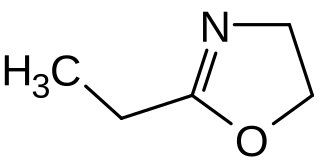
2-Ethyl-2-oxazoline (EtOx) is an oxazoline which is used particularly as a monomer for the cationic ring-opening polymerization to poly(2-alkyloxazoline)s. This type of polymers are under investigation as readily water-soluble and biocompatible materials for biomedical applications.
Parisa Mehrkhodavandi is a Canadian chemist and Professor of Chemistry at the University of British Columbia (UBC). Her research focuses on the design of new catalysts that can effect polymerization of sustainably sourced or biodegradable polymers.

Hafnium(IV) triflate or hafnium trifluoromethansulfonate is an inorganic substance with the idealized formula Hf(OSO2CF3)4, also written as Hf(OTf)4. Hafnium triflate is used as an impure mixture as a catalyst. Hafnium (IV) has an ionic radius of intermediate range (Al < Ti < Hf < Zr < Sc < Ln) and has an oxophilic hard character typical of group IV metals. This solid is a stronger Lewis acid than its typical precursor hafnium tetrachloride, HfCl4, because of the strong electron-withdrawing nature of the four triflate groups, which makes it a great Lewis acid and has many uses including as a great catalyst at low Lewis acid loadings for electrophilic aromatic substitution and nucleophilic substitution reactions.

Poly(trimethylene carbonate) (PTMC) is an aliphatic polycarbonate synthesized from the 6-membered cyclic carbonate, trimethylene carbonate. Trimethylene carbonate (TMC) is a colorless crystalline solid with melting point ranging between 45°C and 48 °C and boiling point at 255ºC. TMC is originally synthesized from 1,3-propanediol with phosgene or carbon monoxide, which are highly poisonous gases. Another route is from the transesterification of 1,3-propanediol and dialkylcarbonates. This route is considered "greener" compared to the other one, since precursors can be obtained from renewable resources and carbon dioxide.





![<span class="mw-page-title-main">Sulfonium</span> Cation of the form [SR3]+](https://upload.wikimedia.org/wikipedia/commons/thumb/6/62/%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png/320px-%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png)









