Passivation, in physical chemistry and engineering, refers to coating a material so it becomes "passive", that is, less readily affected or corroded by the environment. Passivation involves creation of an outer layer of shield material that is applied as a microcoating, created by chemical reaction with the base material, or allowed to build by spontaneous oxidation in the air. As a technique, passivation is the use of a light coat of a protective material, such as metal oxide, to create a shield against corrosion. Passivation of silicon is used during fabrication of microelectronic devices. In electrochemical treatment of water, passivation reduces the effectiveness of the treatment by increasing the circuit resistance, and active measures are typically used to overcome this effect, the most common being polarity reversal, which results in limited rejection of the fouling layer.

A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.

Lead(II) iodide is a chemical compound with the formula PbI
2. At room temperature, it is a bright yellow odorless crystalline solid, that becomes orange and red when heated. It was formerly called plumbous iodide.

Indium(III) oxide (In2O3) is a chemical compound, an amphoteric oxide of indium.
A definition in semiconductor physics, carrier lifetime is defined as the average time it takes for a minority carrier to recombine. The process through which this is done is typically known as minority carrier recombination.
Methylammonium chloride in an organic halide with a formula of CH3NH3Cl. It is an ammonium salt composed of methylamine and hydrogen chloride. One potential application for the methylammonium halides is in the production of perovskite solar cells.

Ruddlesden-Popper (RP) phases are a type of perovskite structure that consists of two-dimensional perovskite-like slabs interleaved with cations. The general formula of an RP phase is An+1BnX3n+1, where A and B are cations, X is an anion, and n is the number of octahedral layers in the perovskite-like stack. Generally, it has a phase structure that results from the intergrowth of perovskite-type and NaCl-type structures.

A perovskite solar cell (PSC) is a type of solar cell that includes a perovskite-structured compound, most commonly a hybrid organic–inorganic lead or tin halide-based material as the light-harvesting active layer. Perovskite materials, such as methylammonium lead halides and all-inorganic caesium lead halide, are cheap to produce and simple to manufacture.
Methylammonium halides are organic halides with a formula of [CH3NH3]+X−, where X is Cl for methylammonium chloride, Br for methylammonium bromide, or I for methylammonium iodide. Generally they are white or light colored powders.

Methylammonium bromide in an organic halide with a formula of CH3NH3Br. It is the salt of methylammonium and bromide. It is a colorless, water-soluble solid.
Methylammonium iodide in an organic halide with a formula of CH3NH3I. It is an ammonium salt composed of methylamine and hydrogen iodide. The primary application for methylammonium iodide, sometimes in combination with other methylammonium halides, is as a component of perovskite (structure) crystalline solar cells.
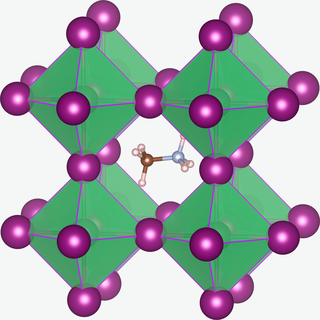
Methylammonium lead halides (MALHs) are solid compounds with perovskite structure and a chemical formula of CH3NH3PbX3, where X = I, Br or Cl. They have potential applications in solar cells, lasers, light-emitting diodes, photodetectors, radiation detectors, scintillator, magneto-optical data storage and hydrogen production.
A tin-based perovskite solar cell is a special type of perovskite solar cell, where the lead is substituted by tin. It has a tin-based perovskite structure (ASnX3), where 'A' is a 1+ cation and 'X' is a monovalent halogen anion. The methylammonium tin triiodide (CH3NH3SnI3) has a band gap of 1.2–1.3 eV, while formamidinium tin triiodide has a band gap of 1.4 eV.

Mercouri Kanatzidis is a Charles E. and Emma H. Morrison Professor of chemistry and professor of materials science and engineering at Northwestern University and Senior Scientist at Argonne National Laboratory.
Arnold Guloy is an American chemist who is Professor of Chemistry at the University of Houston. He is an expert in the area Zintl phases chemistry, crystal growth, materials discovery, and superconductivity.
Laura Maria Herz is a Professor of Physics at the University of Oxford. She works on femtosecond spectroscopy for the analysis of semiconductor materials.

Maksym V. Kovalenko is a full professor of inorganic chemistry and the head of the Functional Inorganic Materials group at ETH Zurich. A part of the research activities of the group are conducted at Empa (Dübendorf). He is working in the fields of solid-state chemistry, quantum dots and other nanomaterials, surface chemistry, self-assembly, optical spectroscopy, optoelectronics and energy storage.

Perovskite nanocrystals are a class of semiconductor nanocrystals, which exhibit unique characteristics that separate them from traditional quantum dots. Perovskite nanocrystals have an ABX3 composition where A = cesium, methylammonium (MA), or formamidinium (FA); B = lead or tin; and X = chloride, bromide, or iodide.

Mohammad Khaja Nazeeruddin is an Indian-Swiss chemist and materials scientist specialized in Perovskite solar cells, dye-sensitized solar cells, and light-emitting diodes. He is a professor at EPFL and the director of the Laboratory for Molecular Engineering of Functional Materials at School of Basic Sciences.

Fred Wudl is an American materials scientist, academic researcher. He is a Professor Emeritus in the Department of Materials Engineering at the University of California, Santa Barbara.












