Related Research Articles
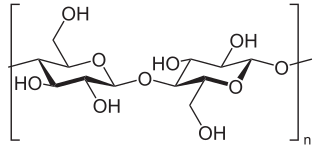
Cellulose is an organic compound with the formula (C
6H
10O
5)
n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms. Cellulose is the most abundant organic polymer on Earth. The cellulose content of cotton fiber is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%.
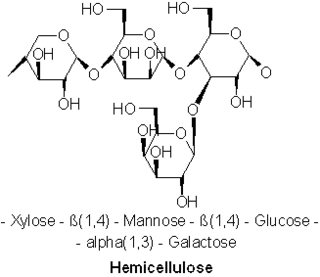
A hemicellulose is one of a number of heteropolymers, such as arabinoxylans, present along with cellulose in almost all terrestrial plant cell walls. Cellulose is crystalline, strong, and resistant to hydrolysis. Hemicelluloses are branched, shorter in length than cellulose, and also show a propensity to crystallize. They can be hydrolyzed by dilute acid or base as well as a myriad of hemicellulase enzymes.

A polymer is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.

Guar gum, also called guaran, is a galactomannan polysaccharide extracted from guar beans that has thickening and stabilizing properties useful in food, feed, and industrial applications. The guar seeds are mechanically dehusked, hydrated, milled and screened according to application. It is typically produced as a free-flowing, off-white powder.

Silica gel is an amorphous and porous form of silicon dioxide (silica), consisting of an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other liquids, or may be filled by gas or vacuum. In the last case, the material is properly called silica xerogel.

Cellulase is any of several enzymes produced chiefly by fungi, bacteria, and protozoans that catalyze cellulolysis, the decomposition of cellulose and of some related polysaccharides:
Excipient is a substance formulated alongside the active ingredient of a medication. Excipients serve various purposes, including long-term stabilization, bulking up solid formulations containing potent active ingredients in small amounts, or enhancing the therapeutic properties of the active ingredient in the final dosage form. They can facilitate drug absorption, reduce viscosity, or enhance solubility. Excipients can also aid in the manufacturing process by improving the handling of active substances, facilitating powder flowability, or preventing denaturation and aggregation during the expected shelf life. The selection of excipients depends on factors such as the route of administration, dosage form, and active ingredient.

Polyvinyl fluoride (PVF) or –(CH2CHF)n– is a polymer material mainly used in the flammability-lowering coatings of airplane interiors and photovoltaic module backsheets. It is also used in raincoats and metal sheeting. Polyvinyl fluoride is a thermoplastic fluoropolymer with a repeating vinyl fluoride unit, and it is structurally very similar to polyvinyl chloride.
Polyamide-imides are either thermosetting or thermoplastic, amorphous polymers that have exceptional mechanical, thermal and chemical resistant properties. Polyamide-imides are used extensively as wire coatings in making magnet wire. They are prepared from isocyanates and TMA in N-methyl-2-pyrrolidone (NMP). A prominent distributor of polyamide-imides is Solvay Specialty Polymers, which uses the trademark Torlon.
An anticaking agent is an additive placed in powdered or granulated materials, such as table salt or confectioneries, to prevent the formation of lumps (caking) and for easing packaging, transport, flowability, and consumption. Caking mechanisms depend on the nature of the material. Crystalline solids often cake by formation of liquid bridge and subsequent fusion of microcrystals. Amorphous materials can cake by glass transitions and changes in viscosity. Polymorphic phase transitions can also induce caking.

A thickening agent or thickener is a substance which can increase the viscosity of a liquid without substantially changing its other properties. Edible thickeners are commonly used to thicken sauces, soups, and puddings without altering their taste; thickeners are also used in paints, inks, explosives, and cosmetics.

Carboxymethyl cellulose (CMC) or cellulose gum is a cellulose derivative with carboxymethyl groups (-CH2-COOH) bound to some of the hydroxyl groups of the glucopyranose monomers that make up the cellulose backbone. It is often used in its sodium salt form, sodium carboxymethyl cellulose. It used to be marketed under the name Tylose, a registered trademark of SE Tylose.

Natural fibers or natural fibres are fibers that are produced by geological processes, or from the bodies of plants or animals. They can be used as a component of composite materials, where the orientation of fibers impacts the properties. Natural fibers can also be matted into sheets to make paper or felt.
A glazing agent is a natural or synthetic substance that provides a waxy, homogeneous coating to prevent water loss from a surface and provide other protection.

Hot-melt adhesive (HMA), also known as hot glue, is a form of thermoplastic adhesive that is commonly sold as solid cylindrical sticks of various diameters designed to be applied using a hot glue gun. The gun uses a continuous-duty heating element to melt the plastic glue, which the user pushes through the gun either with a mechanical trigger mechanism on the gun, or with direct finger pressure. The glue squeezed out of the heated nozzle is initially hot enough to burn and even blister skin. The glue is sticky when hot, and solidifies in a few seconds to one minute. Hot-melt adhesives can also be applied by dipping or spraying, and are popular with hobbyists and crafters both for affixing and as an inexpensive alternative to resin casting.

Hypromellose (INN), short for hydroxypropyl methylcellulose (HPMC), is a semisynthetic, inert, viscoelastic polymer used in eye drops, as well as an excipient and controlled-delivery component in oral medicaments, found in a variety of commercial products.
Dynamic vapor sorption (DVS) is a gravimetric technique that measures how quickly and how much of a solvent is absorbed by a sample such as a dry powder absorbing water. It does this by varying the vapor concentration surrounding the sample and measuring the change in mass which this produces. The technique is mostly used for water vapor, but is suitable for a wide range of organic solvents. Daryl Williams, founder of Surface Measurement Systems Ltd, developed Dynamic Vapor Sorption in 1991; the first instrument was delivered to Pfizer UK in 1992. DVS was originally developed to replace the time and labor-intensive desiccators and saturated salt solutions used to measure water vapor sorption isotherms.

Nanocellulose is a term referring to nano-structured cellulose. This may be either cellulose nanocrystal, cellulose nanofibers (CNF) also called nanofibrillated cellulose (NFC), or bacterial nanocellulose, which refers to nano-structured cellulose produced by bacteria.

Bacterial cellulose is an organic compound with the formula (C
6H
10O
5)
n produced by certain types of bacteria. While cellulose is a basic structural material of most plants, it is also produced by bacteria, principally of the genera Komagataeibacter, Acetobacter, Sarcina ventriculi and Agrobacterium. Bacterial, or microbial, cellulose has different properties from plant cellulose and is characterized by high purity, strength, moldability and increased water holding ability. In natural habitats, the majority of bacteria synthesize extracellular polysaccharides, such as cellulose, which form protective envelopes around the cells. While bacterial cellulose is produced in nature, many methods are currently being investigated to enhance cellulose growth from cultures in laboratories as a large-scale process. By controlling synthesis methods, the resulting microbial cellulose can be tailored to have specific desirable properties. For example, attention has been given to the bacteria Komagataeibacter xylinus due to its cellulose's unique mechanical properties and applications to biotechnology, microbiology, and materials science.
References
- ↑ "Agricultural Marketing Service". Archived from the original on 8 July 2014. Retrieved 9 November 2016.
- ↑ Matrosovich, Mikhail; Tatyana Matrosovich; Wolfgang Garten; Hans-Dieter Klenk (2006). "New low-viscosity overlay medium for viral plaque assays". Virology Journal. 3. BioMed Central: 63. doi: 10.1186/1743-422X-3-63 . PMC 1564390 . PMID 16945126.
- ↑ "FOODS". Archived from the original on 5 March 2016. Retrieved 9 November 2016.
- ↑ Decorative skin and hair cosmetics containing microcrystalline cellulose as enhancing agent, 2004-01-06, retrieved 2018-05-21
- ↑ "Microcrystalline Cellulose | Cosmetics Info". www.cosmeticsinfo.org. Retrieved 2018-05-21.
- ↑ "Hindi, S. S. Z. 2016. Microcrystalline cellulose: Its specifications and pharmaceutical processing. Biocrystals Journal. 1 (1): 26-38". BioCrystals. Archived from the original on 2016-05-08. Retrieved 2016-04-22.
- ↑ "Letter from Allergy Clinic of Tulsa". Archived from the original on 2017-03-23. Retrieved 2012-02-03.
- ↑ https://www.mastcellaction.org/assets/_/2021/09/13/29fd2269-847a-4ab3-9295-d6432316233a/recognition-and-management-of-excipient-reactivity-in-mast-cell-activation-syndrome.pdf?v=1