Related Research Articles

Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bind to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids (DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere.

A hybrid-propellant rocket is a rocket with a rocket motor that uses rocket propellants in two different phases: one solid and the other either gas or liquid. The hybrid rocket concept can be traced back to the early 1930s.

Hydrazine is an inorganic compound with the chemical formula N2H4. It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour.

A hypergolic propellant combination used in a rocket engine is one whose components spontaneously ignite when they come into contact with each other.

Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russia rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is 92.011 g/mol.

Red fuming nitric acid (RFNA) is a storable oxidizer used as a rocket propellant. It consists of 84% nitric acid, 13% dinitrogen tetroxide and 1–2% water. The color of red fuming nitric acid is due to the dinitrogen tetroxide, which breaks down partially to form nitrogen dioxide. The nitrogen dioxide dissolves until the liquid is saturated, and produces toxic fumes with a suffocating odor. RFNA increases the flammability of combustible materials and is highly exothermic when reacting with water.

Nitrogen dioxide is a chemical compound with the formula NO
2. It is one of several nitrogen oxides. NO
2 is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year for use primarily in the production of fertilizers. At higher temperatures it is a reddish-brown gas. It can be fatal if inhaled in large quantities. Nitrogen dioxide is a paramagnetic, bent molecule with C2v point group symmetry.
Monomethylhydrazine is a highly toxic, volatile hydrazine derivative with the chemical formula CH3NHNH2. It is used as a rocket propellant in bipropellant rocket engines because it is hypergolic with various oxidizers such as nitrogen tetroxide and nitric acid. As a propellant, it is described in specification MIL-PRF-27404.

Liquid oxygen—abbreviated LOx, LOX or Lox in the aerospace, submarine and gas industries—is the liquid form of molecular oxygen. It was used as the oxidizer in the first liquid-fueled rocket invented in 1926 by Robert H. Goddard, an application which has continued to the present.

A liquid-propellant rocket or liquid rocket utilizes a rocket engine that uses liquid propellants. Liquids are desirable because they have a reasonably high density and high specific impulse (Isp). This allows the volume of the propellant tanks to be relatively low. It is also possible to use lightweight centrifugal turbopumps to pump the rocket propellant from the tanks into the combustion chamber, which means that the propellants can be kept under low pressure. This permits the use of low-mass propellant tanks that do not need to resist the high pressures needed to store significant amounts of gasses, resulting in a low mass ratio for the rocket.
Aerozine 50 is a 50:50 mix by weight of hydrazine and unsymmetrical dimethylhydrazine (UDMH), originally developed in the late 1950s by Aerojet General Corporation as a storable, high-energy, hypergolic fuel for the Titan II ICBM rocket engines. Aerozine continues in wide use as a rocket fuel, typically with dinitrogen tetroxide as the oxidizer, with which it is hypergolic. Aerozine 50 is more stable than hydrazine alone, and has a higher density and boiling point than UDMH alone.

The Titan IIIC was an expendable launch system used by the United States Air Force from 1965 until 1982. It was the first Titan booster to feature large solid rocket motors and was planned to be used as a launcher for the Dyna-Soar, though the spaceplane was cancelled before it could fly. The majority of the launcher's payloads were DoD satellites, for military communications and early warning, though one flight (ATS-6) was performed by NASA. The Titan IIIC was launched exclusively from Cape Canaveral while its sibling, the Titan IIID, was launched only from Vandenberg AFB.
The highest specific impulse chemical rockets use liquid propellants. They can consist of a single chemical or a mix of two chemicals, called bipropellants. Bipropellants can further be divided into two categories; hypergolic propellants, which ignite when the fuel and oxidizer make contact, and non-hypergolic propellants which require an ignition source.

Hydroxylammonium nitrate or hydroxylamine nitrate (HAN) is an inorganic compound with the chemical formula [NH3OH][NO3]. It is a salt derived from hydroxylamine and nitric acid. In its pure form, it is a colourless hygroscopic solid. It has potential to be used as a rocket propellant either as a solution in monopropellants or bipropellants. Hydroxylammonium nitrate (HAN) based propellants are a viable and effective solution for future green propellant based missions, as it offers 50% higher performance for a given propellant tank compared to commercially used hydrazine.

The AJ10 is a hypergolic rocket engine manufactured by Aerojet Rocketdyne. It has been used to propel the upper stages of several launch vehicles, including the Delta II and Titan III. Variants were and are used as the service propulsion engine for the Apollo command and service module, in the Space Shuttle Orbital Maneuvering System, and on NASA's Orion spacecraft.
Nitrous oxide fuel blend propellants are a class of liquid rocket propellants that were intended in the early 2010s to be able to replace hydrazine as the standard storable rocket propellent in some applications.

Rocket propellant is the reaction mass of a rocket. This reaction mass is ejected at the highest achievable velocity from a rocket engine to produce thrust. The energy required can either come from the propellants themselves, as with a chemical rocket, or from an external source, as with ion engines.

A liquid apogee engine (LAE), or apogee engine, refers to a type of chemical rocket engine typically used as the main engine in a spacecraft.
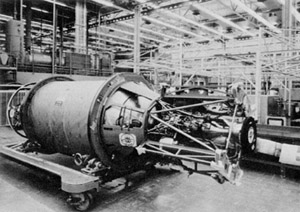
The Bell Aerosystems Company XLR81 was an American liquid-propellant rocket engine, which was used on the Agena upper stage. It burned UDMH and RFNA fed by a turbopump in a fuel rich gas generator cycle. The turbopump had a single turbine with a gearbox to transmit power to the oxidizer and fuel pumps. The thrust chamber was all-aluminum, and regeneratively cooled by oxidizer flowing through gun-drilled passages in the combustion chamber and throat walls. The nozzle was a titanium radiatively cooled extension. The engine was mounted on a hydraulic actuated gimbal which enabled thrust vectoring to control pitch and yaw. Engine thrust and mixture ratio were controlled by cavitating flow venturis on the gas generator flow circuit. Engine start was achieved by solid propellant start cartridge.
References
- ↑ Wright, Alfred C. (February 1977). USAF Propellant Handbooks. Nitric Acid/Nitrogen Tetroxide Oxidizers. Volume II. USAF (Report). Defense Technical Information Center. AFRPL-TR-76-76 (DTIC Accession Number ADA036741). Archived from the original on March 3, 2016. Retrieved 14 April 2013.
- ↑ Robert A. Braeunig (2008). "Rocket Propellants". Rocket and Space Technology. Archived from the original on 21 May 2013. Retrieved 14 April 2013.