Filtration is a physical separation process that separates solid matter and fluid from a mixture using a filter medium that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. Oversize particles may form a filter cake on top of the filter and may also block the filter lattice, preventing the fluid phase from crossing the filter, known as blinding. The size of the largest particles that can successfully pass through a filter is called the effective pore size of that filter. The separation of solid and fluid is imperfect; solids will be contaminated with some fluid and filtrate will contain fine particles. Filtration occurs both in nature and in engineered systems; there are biological, geological, and industrial forms.

In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when one of the solvents is water.

A solvent is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell.

Solvation describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the solute, including solubility, reactivity, and color, as well as influencing the properties of the solvent such as its viscosity and density. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. The surrounded solute particles then move away from the solid solute and out into the solution. Ions are surrounded by a concentric shell of solvent. Solvation is the process of reorganizing solvent and solute molecules into solvation complexes and involves bond formation, hydrogen bonding, and van der Waals forces. Solvation of a solute by water is called hydration.

In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent solubility product which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios.
In physical chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium. Most commonly the term is applied to a solution of a solid in a liquid. A supersaturated solution is in a metastable state; it may be brought to equilibrium by forcing the excess of solute to separate from the solution. The term can also be applied to a mixture of gases.

In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a supersaturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant.
The Bayer process is the principal industrial means of refining bauxite to produce alumina (aluminium oxide) and was developed by Carl Josef Bayer. Bauxite, the most important ore of aluminium, contains only 30–60% aluminium oxide (Al2O3), the rest being a mixture of silica, various iron oxides, and titanium dioxide. The aluminium oxide must be further purified before it can be refined into aluminium metal.

Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, and in the case of liquid crystals, time of fluid evaporation.

Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = C6H5). This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallizing of chemical compounds.
Chiral resolution, or enantiomeric resolution, is a process in stereochemistry for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active compounds, including drugs. Another term with the same meaning is optical resolution.

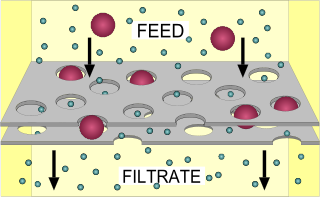
In chemical engineering, biochemical engineering and protein purification, crossflow filtration is a type of filtration. Crossflow filtration is different from dead-end filtration in which the feed is passed through a membrane or bed, the solids being trapped in the filter and the filtrate being released at the other end. Cross-flow filtration gets its name because the majority of the feed flow travels tangentially across the surface of the filter, rather than into the filter. The principal advantage of this is that the filter cake is substantially washed away during the filtration process, increasing the length of time that a filter unit can be operational. It can be a continuous process, unlike batch-wise dead-end filtration.

Hydrothermal synthesis includes the various techniques of crystallizing substances from high-temperature aqueous solutions at high vapor pressures; also termed "hydrothermal method". The term "hydrothermal" is of geologic origin. Geochemists and mineralogists have studied hydrothermal phase equilibria since the beginning of the twentieth century. George W. Morey at the Carnegie Institution and later, Percy W. Bridgman at Harvard University did much of the work to lay the foundations necessary to containment of reactive media in the temperature and pressure range where most of the hydrothermal work is conducted.
Lithium nitrite is the lithium salt of nitrous acid, with formula LiNO2. This compound is hygroscopic and very soluble in water. It is used as a corrosion inhibitor in mortar. It is also used in the production of explosives, due to its ability to nitrosate ketones under certain conditions.

In chemistry, recrystallization is a technique used to purify chemicals. By dissolving a mixture of a compound and impurities in an appropriate solvent, either the desired compound or impurities can be removed from the solution, leaving the other behind. It is named for the crystals often formed when the compound precipitates out. Alternatively, recrystallization can refer to the natural growth of larger ice crystals at the expense of smaller ones.

Protein crystallization is the process of formation of a regular array of individual protein molecules stabilized by crystal contacts. If the crystal is sufficiently ordered, it will diffract. Some proteins naturally form crystalline arrays, like aquaporin in the lens of the eye.

A sugar cane mill is a factory that processes sugar cane to produce raw sugar or plantation white sugar. Some sugar mills are situated next to a back-end refinery, that turns raw sugar into (refined) white sugar.
In chemistry, cosolvents are substances added to a primary solvent in small amounts to increase the solubility of a poorly-soluble compound. Their use is most prevalent in chemical and biological research relating to pharmaceuticals and food science, where alcohols are frequently used as cosolvents in water to dissolve hydrophobic molecules during extraction, screening, and formulation. Cosolvents find applications also in environmental chemistry and are known as effective countermeasures against pollutant non-aqueous phase liquids, as well as in the production of functional energy materials and synthesis of biodiesel.












