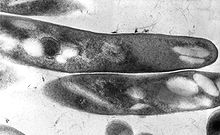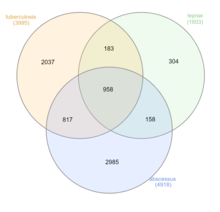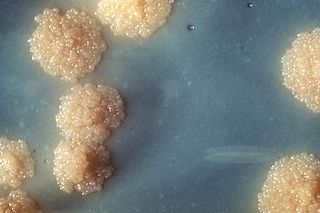
Mycobacterium tuberculosis, also known as Koch's bacillus, is a species of pathogenic bacteria in the family Mycobacteriaceae and the causative agent of tuberculosis. First discovered in 1882 by Robert Koch, M. tuberculosis has an unusual, waxy coating on its cell surface primarily due to the presence of mycolic acid. This coating makes the cells impervious to Gram staining, and as a result, M. tuberculosis can appear weakly Gram-positive. Acid-fast stains such as Ziehl–Neelsen, or fluorescent stains such as auramine are used instead to identify M. tuberculosis with a microscope. The physiology of M. tuberculosis is highly aerobic and requires high levels of oxygen. Primarily a pathogen of the mammalian respiratory system, it infects the lungs. The most frequently used diagnostic methods for tuberculosis are the tuberculin skin test, acid-fast stain, culture, and polymerase chain reaction.
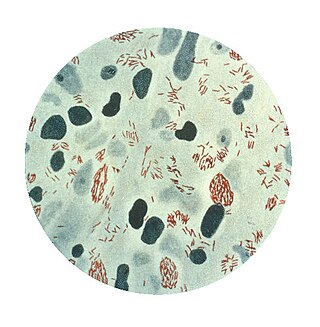
Mycobacterium leprae is one of the two species of bacteria that cause Hansen's disease (leprosy), a chronic but curable infectious disease that damages the peripheral nerves and targets the skin, eyes, nose, and muscles.
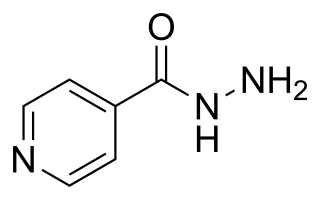
Isoniazid, also known as isonicotinic acid hydrazide (INH), is an antibiotic used for the treatment of tuberculosis. For active tuberculosis, it is often used together with rifampicin, pyrazinamide, and either streptomycin or ethambutol. For latent tuberculosis, it is often used alone. It may also be used for atypical types of mycobacteria, such as M. avium, M. kansasii, and M. xenopi. It is usually taken by mouth, but may be used by injection into muscle.
Nontuberculous mycobacteria (NTM), also known as environmental mycobacteria, atypical mycobacteria and mycobacteria other than tuberculosis (MOTT), are mycobacteria which do not cause tuberculosis or leprosy/Hansen's disease. NTM are able to cause pulmonary diseases that resemble tuberculosis. Mycobacteriosis is any of these illnesses, usually meant to exclude tuberculosis. They occur in many animals, including humans and are commonly found in soil and water.
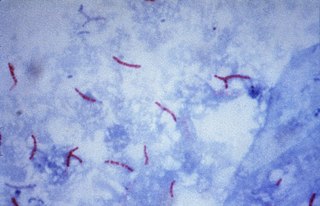
The Ziehl-Neelsen stain, also known as the acid-fast stain, is a bacteriological staining technique used in cytopathology and microbiology to identify acid-fast bacteria under microscopy, particularly members of the Mycobacterium genus. This staining method was initially introduced by Paul Ehrlich (1854–1915) and subsequently modified by the German bacteriologists Franz Ziehl (1859–1926) and Friedrich Neelsen (1854–1898) during the late 19th century.

Mycobacterium smegmatis is an acid-fast bacterial species in the phylum Actinomycetota and the genus Mycobacterium. It is 3.0 to 5.0 μm long with a bacillus shape and can be stained by Ziehl–Neelsen method and the auramine-rhodamine fluorescent method. It was first reported in November 1884 by Lustgarten, who found a bacillus with the staining appearance of tubercle bacilli in syphilitic chancres. Subsequent to this, Alvarez and Tavel found organisms similar to that described by Lustgarten also in normal genital secretions (smegma). This organism was later named M. smegmatis.

Mycobacterium avium-intracellulare infection (MAI) is an atypical mycobacterial infection, i.e. one with nontuberculous mycobacteria or NTM, caused by Mycobacterium avium complex (MAC), which is made of two Mycobacterium species, M. avium and M. intracellulare. This infection causes respiratory illness in birds, pigs, and humans, especially in immunocompromised people. In the later stages of AIDS, it can be very severe. It usually first presents as a persistent cough. It is typically treated with a series of three antibiotics for a period of at least six months.

Ethionamide is an antibiotic used to treat tuberculosis. Specifically it is used, along with other antituberculosis medications, to treat active multidrug-resistant tuberculosis. It is no longer recommended for leprosy. It is taken by mouth.

A mycobacteriophage is a member of a group of bacteriophages known to have mycobacteria as host bacterial species. While originally isolated from the bacterial species Mycobacterium smegmatis and Mycobacterium tuberculosis, the causative agent of tuberculosis, more than 4,200 mycobacteriophage have since been isolated from various environmental and clinical sources. 2,042 have been completely sequenced. Mycobacteriophages have served as examples of viral lysogeny and of the divergent morphology and genetic arrangement characteristic of many phage types.
The Timpe and Runyon classification of nontuberculous mycobacteria based on the rate of growth, production of yellow pigment and whether this pigment was produced in the dark or only after exposure to light.

Mycobacteroides abscessus is a species of rapidly growing, multidrug-resistant, nontuberculous mycobacteria (NTM) that is a common soil and water contaminant. Although M. abscessus most commonly causes chronic lung infection and skin and soft tissue infection (SSTI), it can also cause infection in almost all human organs, mostly in patients with suppressed immune systems. Amongst NTM species responsible for disease, infection caused by M. abscessus complex are more difficult to treat due to antimicrobial drug resistance.

Mycobacterium fortuitum is a nontuberculous species of the phylum Actinomycetota, belonging to the genus Mycobacterium.
Mycobacterium gordonae is a species of Mycobacterium named for Ruth E. Gordon. It is a species of the phylum Actinomycetota, belonging to the genus Mycobacterium.
Mycobacterium avium complex is a group of mycobacteria comprising Mycobacterium intracellulare and Mycobacterium avium that are commonly grouped because they infect humans together; this group, in turn, is part of the group of nontuberculous mycobacteria. These bacteria cause Mycobacterium avium-intracellulare infections or Mycobacterium avium complex infections in humans. These bacteria are common and are found in fresh and salt water, in household dust and in soil. MAC bacteria usually cause infection in those who are immunocompromised or those with severe lung disease.

Mycobacterium kansasii is a bacterium in the Mycobacterium genus. It is an environmental bacteria that causes opportunistic infections in humans, and is one of the leading mycobacterial causes of human disease after tuberculosis and leprosy.

Cord factor, or trehalose dimycolate (TDM), is a glycolipid molecule found in the cell wall of Mycobacterium tuberculosis and similar species. It is the primary lipid found on the exterior of M. tuberculosis cells. Cord factor influences the arrangement of M. tuberculosis cells into long and slender formations, giving its name. Cord factor is virulent towards mammalian cells and critical for survival of M. tuberculosis in hosts, but not outside of hosts. Cord factor has been observed to influence immune responses, induce the formation of granulomas, and inhibit tumor growth. The antimycobacterial drug SQ109 is thought to inhibit TDM production levels and in this way disrupts its cell wall assembly.
Mycobacterium scrofulaceum is a species of Mycobacterium.
Mycobacterium lepromatosis is an aerobic, acid-fast bacillus (AFB), and the second known causative agent of Hansen's disease (leprosy). It was discovered in 2008. Analysis of the 16S rRNA gene confirms that the species is distinct from Mycobacterium leprae.

A lung cavity or pulmonary cavity is an abnormal, thick-walled, air-filled space within the lung. Cavities in the lung can be caused by infections, cancer, autoimmune conditions, trauma, congenital defects, or pulmonary embolism. The most common cause of a single lung cavity is lung cancer. Bacterial, mycobacterial, and fungal infections are common causes of lung cavities. Globally, tuberculosis is likely the most common infectious cause of lung cavities. Less commonly, parasitic infections can cause cavities. Viral infections almost never cause cavities. The terms cavity and cyst are frequently used interchangeably; however, a cavity is thick walled, while a cyst is thin walled. The distinction is important because cystic lesions are unlikely to be cancer, while cavitary lesions are often caused by cancer.
Mycobacteroides franklinii is a species of bacteria from the phylum Actinomycetota belonging to the genus Mycobacteroides. Most of the original strains were isolated from clinical specimens in Pennsylvania, but some have been found in conduit water in the Netherlands. In general, human M. franklinii infections present with symptoms similar to an infection with Mycobacteroides abscessus, but it can also be associated with tattoo infections. M. franklinii is also associated with outbreaks of mycobacteriosis in farmed fish. M. fanklinii is susceptible to cefoxitin and bedaquiline.