
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis. This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substances are considered carcinogens, but their carcinogenic activity is attributed to the radiation, for example gamma rays and alpha particles, which they emit. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise from both natural and synthetic substances. Carcinogens are not necessarily immediately toxic; thus, their effect can be insidious.

In genetics, a mutagen is a physical or chemical agent that permanently changes genetic material, usually DNA, in an organism and thus increases the frequency of mutations above the natural background level. As many mutations can cause cancer in animals, such mutagens can therefore be carcinogens, although not all necessarily are. All mutagens have characteristic mutational signatures with some chemicals becoming mutagenic through cellular processes.
Teratology is the study of abnormalities of physiological development in organisms during their life span. It is a sub-discipline in medical genetics which focuses on the classification of congenital abnormalities in dysmorphology caused by teratogens. Teratogens are substances that may cause non-heritable birth defects via a toxic effect on an embryo or fetus. Defects include malformations, disruptions, deformations, and dysplasia that may cause stunted growth, delayed mental development, or other congenital disorders that lack structural malformations. The related term developmental toxicity includes all manifestations of abnormal development that are caused by environmental insult. The extent to which teratogens will impact an embryo is dependent on several factors, such as how long the embryo has been exposed, the stage of development the embryo was in when exposed, the genetic makeup of the embryo, and the transfer rate of the teratogen.

Trichloroethylene (TCE) is a halocarbon with the formula C2HCl3, commonly used as an industrial degreasing solvent. It is a clear, colourless, non-flammable, volatile liquid with a chloroform-like pleasant mild smell and sweet taste. Its IUPAC name is trichloroethene. Trichloroethylene has been sold under a variety of trade names. Industrial abbreviations include TCE, trichlor, Trike, Tricky and tri. Under the trade names Trimar and Trilene, it was used as a volatile anesthetic and as an inhaled obstetrical analgesic. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as chlorothene.

A birth defect, also known as a congenital disorder, is an abnormal condition that is present at birth regardless of its cause. Birth defects may result in disabilities that may be physical, intellectual, or developmental. The disabilities can range from mild to severe. Birth defects are divided into two main types: structural disorders in which problems are seen with the shape of a body part and functional disorders in which problems exist with how a body part works. Functional disorders include metabolic and degenerative disorders. Some birth defects include both structural and functional disorders.

MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is an organic compound. It is classified as a tetrahydropyridine. It is of interest as a precursor to the neurotoxin MPP+, which causes permanent symptoms of Parkinson's disease by destroying dopaminergic neurons in the substantia nigra of the brain. It has been used to study disease models in various animals.
Genotoxicity is the property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, but some genotoxic substances are not mutagenic. The alteration can have direct or indirect effects on the DNA: the induction of mutations, mistimed event activation, and direct DNA damage leading to mutations. The permanent, heritable changes can affect either somatic cells of the organism or germ cells to be passed on to future generations. Cells prevent expression of the genotoxic mutation by either DNA repair or apoptosis; however, the damage may not always be fixed leading to mutagenesis.

Methylcholanthrene is a highly carcinogenic polycyclic aromatic hydrocarbon produced by burning organic compounds at very high temperatures. Methylcholanthrene is also known as 3-methylcholanthrene, 20-methylcholanthrene or the IUPAC name 3-methyl-1,2-dyhydrobenzo[j]aceanthrylene. The short notation often used is 3-MC or MCA. This compound forms pale yellow solid crystals when crystallized from benzene and ether. It has a melting point around 180 °C and its boiling point is around 280 °C at a pressure of 80 mmHg. Methylcholanthrene is used in laboratory studies of chemical carcinogenesis. It is an alkylated derivative of benz[a]anthracene and has a similar UV spectrum. The most common isomer is 3-methylcholanthrene, although the methyl group can occur in other places.
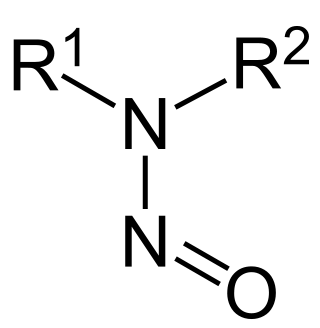
In organic chemistry, nitrosamines are organic compounds with the chemical structure R2N−N=O, where R is usually an alkyl group. They feature a nitroso group bonded to a deprotonated amine. Most nitrosamines are carcinogenic in nonhuman animals. A 2006 systematic review supports a "positive association between nitrite and nitrosamine intake and gastric cancer, between meat and processed meat intake and gastric cancer and oesophageal cancer, and between preserved fish, vegetable and smoked food intake and gastric cancer, but is not conclusive".
Environmental toxicants and fetal development is the impact of different toxic substances from the environment on the development of the fetus. This article deals with potential adverse effects of environmental toxicants on the prenatal development of both the embryo or fetus, as well as pregnancy complications. The human embryo or fetus is relatively susceptible to impact from adverse conditions within the mother's environment. Substandard fetal conditions often cause various degrees of developmental delays, both physical and mental, for the growing baby. Although some variables do occur as a result of genetic conditions pertaining to the father, a great many are directly brought about from environmental toxins that the mother is exposed to.
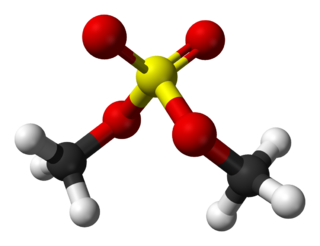
Dimethyl sulfate (DMS) is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as (CH3)2SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agent in organic synthesis.
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted by rice plantations in small amounts. It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans, and in lesser amounts on land by terrestrial fungi and bacteria. It is used in organic synthesis as a source of methyl groups.

Ochratoxin A—a toxin produced by different Aspergillus and Penicillium species — is one of the most-abundant food-contaminating mycotoxins. It is also a frequent contaminant of water-damaged houses and of heating ducts. Human exposure can occur through consumption of contaminated food products, particularly contaminated grain and pork products, as well as coffee, wine grapes, and dried grapes. The toxin has been found in the tissues and organs of animals, including human blood and breast milk. Ochratoxin A, like most toxic substances, has large species- and sex-specific toxicological differences.

Nitrosourea is both the name of a molecule, and a class of compounds that include a nitroso (R-NO) group and a urea.

Chloromethyl methyl ether (CMME) is a compound with formula CH3OCH2Cl. A colorless liquid, it is a chloroalkyl ether. It is used as an alkylating agent. In organic synthesis, it is used for introducing the methoxymethyl ether (MOM) protecting group, and is thus often called MOM-Cl or MOM chloride. It also finds application as a chloromethylating agent in some variants of the Blanc chloromethylation.
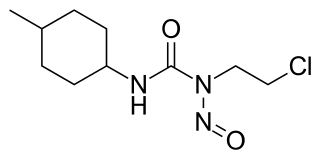
Semustine is an alkylating nitrosourea compound used in chemotherapy treatment of various types of tumours. Due to its lipophilic property, semustine can cross the blood-brain barrier for the chemotherapy of brain tumours, where it interferes with DNA replication in the rapidly-dividing tumour cells. Semustine, just as lomustine, is administered orally. Evidence has been found that treatment with semustine can cause acute leukaemia as a delayed effect in very rare cases.

Reproductive toxicity refers to the potential risk from a given chemical, physical or biologic agent to adversely affect both male and female fertility as well as offspring development. Reproductive toxicants may adversely affect sexual function, ovarian failure, fertility as well as causing developmental toxicity in the offspring. Lowered effective fertility related to reproductive toxicity relates to both male and female effects alike and is reflected in decreased sperm counts, semen quality and ovarian failure. Infertility is medically defined as a failure of a couple to conceive over the course of one year of unprotected intercourse. As many as 20% of couples experience infertility. Among men, oligospermia is defined as a paucity of viable spermatozoa in the semen, whereas azoospermia refers to the complete absence of viable spermatozoa in the semen.

Aflatoxin B1 is an aflatoxin produced by Aspergillus flavus and A. parasiticus. It is a very potent carcinogen with a TD50 3.2 μg/kg/day in rats. This carcinogenic potency varies across species with some, such as rats and monkeys, seemingly much more susceptible than others. Aflatoxin B1 is a common contaminant in a variety of foods including peanuts, cottonseed meal, corn, and other grains; as well as animal feeds. Aflatoxin B1 is considered the most toxic aflatoxin and it is highly implicated in hepatocellular carcinoma (HCC) in humans. In animals, aflatoxin B1 has also been shown to be mutagenic, teratogenic, and to cause immunosuppression. Several sampling and analytical methods including thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), mass spectrometry, and enzyme-linked immunosorbent assay (ELISA), among others, have been used to test for aflatoxin B1 contamination in foods. According to the Food and Agriculture Organization (FAO), a division of the United Nations, the worldwide maximum tolerated levels of aflatoxin B1 was reported to be in the range of 1–20 μg/kg (or .001 ppm - 1 part-per-billion) in food, and 5–50 μg/kg (.005 ppm) in dietary cattle feed in 2003.

Developmental toxicity is any developmental malformation that is caused by the toxicity of a chemical or pathogen. It is the structural or functional alteration, reversible or irreversible, which interferes with homeostasis, normal growth, differentiation, development or behavior. Developmental toxicity is caused by environmental insult, which includes drugs, alcohol, diet, toxic chemicals, and physical factors.

PhIP (2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) is one of the most abundant heterocyclic amines (HCAs) in cooked meat. PhIP is formed at high temperatures from the reaction between creatine or creatinine, amino acids, and sugar. PhIP formation increases with the temperature and duration of cooking and also depends on the method of cooking and the variety of meat being cooked. The U.S. Department of Health and Human Services National Toxicology Program has declared PhIP as "reasonably anticipated to be a human carcinogen". International Agency for Research on Cancer (IARC), part of World Health Organization, has classified PhIP as IARC Group 2B carcinogen. There is sufficient evidence in experimental animals, as well as in vitro models, for the carcinogenicity of PhIP.

















