
The National Institutes of Health is the primary agency of the United States government responsible for biomedical and public health research. It was founded in the late 1880s and is now part of the United States Department of Health and Human Services. The majority of NIH facilities are located in Bethesda, Maryland, and other nearby suburbs of the Washington metropolitan area, with other primary facilities in the Research Triangle Park in North Carolina and smaller satellite facilities located around the United States. The NIH conducts its own scientific research through the NIH Intramural Research Program (IRP) and provides major biomedical research funding to non-NIH research facilities through its Extramural Research Program.

Science and technology in the United States has a long history, producing many important figures and developments in the field. The United States of America came into being around the Age of Enlightenment, an era in Western philosophy in which writers and thinkers, rejecting the perceived superstitions of the past, instead chose to emphasize the intellectual, scientific and cultural life, centered upon the 18th century, in which reason was advocated as the primary source for legitimacy and authority. Enlightenment philosophers envisioned a "republic of science," where ideas would be exchanged freely and useful knowledge would improve the lot of all citizens.
Technology transfer (TT), also called transfer of technology (TOT), is the process of transferring (disseminating) technology from the person or organization that owns or holds it to another person or organization, in an attempt to transform inventions and scientific outcomes into new products and services that benefit society. Technology transfer is closely related to knowledge transfer.

The Bayh–Dole Act or Patent and Trademark Law Amendments Act is United States legislation permitting ownership by contractors of inventions arising from federal government-funded research. Sponsored by two senators, Birch Bayh of Indiana and Bob Dole of Kansas, the Act was adopted in 1980, is codified at 94 Stat. 3015, and in 35 U.S.C. § 200–212, and is implemented by 37 C.F.R. 401 for federal funding agreements with contractors and 37 C.F.R 404 for licensing of inventions owned by the federal government.

The National Renewable Energy Laboratory (NREL) in the US specializes in the research and development of renewable energy, energy efficiency, energy systems integration, and sustainable transportation. NREL is a federally funded research and development center sponsored by the Department of Energy and operated by the Alliance for Sustainable Energy, a joint venture between MRIGlobal and Battelle. Located in Golden, Colorado, NREL is home to the National Center for Photovoltaics, the National Bioenergy Center, and the National Wind Technology Center.
The Export Administration Regulations (EAR) are a set of regulations found at 15 C.F.R. § 730 et seq. They are administered by the Bureau of Industry and Security, which is part of the US Commerce Department. The EAR regulates export and export restrictions: whether a person may export something from the U.S.; re-export something from a foreign country; or transfer something from one person to another in a foreign country. The EAR apply to physical objects - sometimes referred to as "commodities" - as well as intellectual property such as technology and software.

Medical research, also known as experimental medicine, encompasses a wide array of research, extending from "basic research", – involving fundamental scientific principles that may apply to a preclinical understanding – to clinical research, which involves studies of people who may be subjects in clinical trials. Within this spectrum is applied research, or translational research, conducted to expand knowledge in the field of medicine.

James Packard Love is the director of Knowledge Ecology International, formerly known as the Consumer Project on Technology, a non-governmental organization with offices in Washington, D.C. and Geneva, that works mainly on matters concerning knowledge management and governance, including intellectual property policy and practice and innovation policy, particularly as they relate to health care and access to knowledge.

The National Institute of Biomedical Imaging and Bioengineering (NIBIB), founded at the National Institutes of Health (NIH) in 2000, is located in Bethesda, Maryland. It is one of 27 institutes and centers that are part of NIH, an agency of the U.S. Department of Health and Human Services (HHS).
The Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF) is a global non-profit organization created by Congress in 1983.
In the United States, a cooperative research and development agreement is an agreement between a government agency and a private company or university to work together on research and development.
On 29 March 2010, the US District Court for the Southern District of New York found several of the patent claims on the BRCA1 and BRCA2 breast cancer genes held by Myriad Genetics to be invalid. The patents were initially issued on the basis that the genes were isolated and purified to a non-naturally occurring state, however the court found, amongst other things, that the purification was not markedly different from a product of nature and thus was not patentable. The ruling may have implications for holders of other gene patents and the patentability of other naturally occurring substances. It has the potential to directly affect the operation of the healthcare and medical research industries, particularly with regards to cancer treatment and prevention, and may alter the accessibility of such therapies to patients.
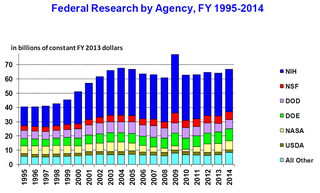
The science policy of the United States is the responsibility of many organizations throughout the federal government. Much of the large-scale policy is made through the legislative budget process of enacting the yearly federal budget, although there are other legislative issues that directly involve science, such as energy policy, climate change, and stem cell research. Further decisions are made by the various federal agencies which spend the funds allocated by Congress, either on in-house research or by granting funds to outside organizations and researchers.
Stanford University v. Roche Molecular Systems, Inc., 563 U.S. 776 (2011), was a United States Supreme Court case in which the Court held that title in a patented invention vests first in the inventor, even if the inventor is a researcher at a federally funded lab subject to the 1980 Bayh–Dole Act. The judges affirmed the common understanding of U.S. constitutional law that inventors originally own inventions they make, and contractual obligations to assign those rights to third parties are secondary.

The NIH Intramural Research Program (IRP) is the internal research program of the National Institutes of Health (NIH), known for its synergistic approach to biomedical science. With 1,200 Principal Investigators and over 4,000 Postdoctoral Fellows conducting basic, translational, and clinical research, the NIH Intramural Research Program is the largest biomedical research institution on earth. The unique funding environment of the IRP facilitates opportunities to conduct both long-term and high-impact science that would otherwise be difficult to undertake. With rigorous external reviews ensuring that only the most outstanding research secures funding, the IRP is responsible for many scientific accomplishments, including the discovery of fluoride to prevent tooth decay, the use of lithium to manage bipolar disorder, and the creation of vaccines against hepatitis, Hemophilus influenzae (Hib), and human papillomavirus (HPV). In addition, the IRP has also produced or trained 21 Nobel Prize-winning scientists.

LifeArc is a British life science medical research charity. It was established in 2000 as MRC Technology to translate the work of UK Medical Research Council (MRC) research scientists.
A biological patent is a patent on an invention in the field of biology that by law allows the patent holder to exclude others from making, using, selling, or importing the protected invention for a limited period of time. The scope and reach of biological patents vary among jurisdictions, and may include biological technology and products, genetically modified organisms and genetic material. The applicability of patents to substances and processes wholly or partially natural in origin is a subject of debate.

Kannalife Sciences Inc., a subsidiary of Neuropathix, Inc., is a bio-pharmaceutical and phyto-medical company based in Doylestown, Pennsylvania founded by Dean Petkanas and Thoma Kikis. Kannalife was formed in 2010 and is involved in the research and development of novel new therapeutic agents designed to reduce oxidative stress, and act as immuno-modulators and neuroprotectants.

Wendy A. Henderson is an American nurse practitioner, scientist, and academic administrator working as the director of the Center of Nursing Scholarship and Innovation at the University of Connecticut. She was previously a clinical investigator and lab chief of the National Institute of Nursing Research digestive disorders unit. Henderson is a Fellow of the American Academy of Nursing.

Mojdeh Bahar is an American patent attorney and government official specialized in technology transfer. She is the associate director for innovation and industry services at the National Institute of Standards and Technology. Bahar previously worked as the assistant administrator for technology transfer at the Agricultural Research Service. She was chief of the cancer branch in the NIH Office of Technology Transfer.












