
Cardiac glycosides are a class of organic compounds that increase the output force of the heart and decrease its rate of contractions by inhibiting the cellular sodium-potassium ATPase pump. Their beneficial medical uses are as treatments for congestive heart failure and cardiac arrhythmias; however, their relative toxicity prevents them from being widely used. Most commonly found as secondary metabolites in several plants such as foxglove plants, these compounds nevertheless have a diverse range of biochemical effects regarding cardiac cell function and have also been suggested for use in cancer treatment.

Erysimum, or wallflower, is a genus of flowering plants in the cabbage family, Brassicaceae. It includes more than 150 species, both popular garden plants and many wild forms. The genus Cheiranthus is sometimes included here in whole or in part. Erysimum has since the early 21st century been ascribed to a monogeneric cruciferous tribe, Erysimeae, characterised by sessile, stellate (star-shaped) and/or malpighiaceous (two-sided) trichomes, yellow to orange flowers and multiseeded siliques.

Asclepias tuberosa, commonly known as butterfly weed, is a species of milkweed native to eastern and southwestern North America. It is commonly known as butterfly weed because of the butterflies that are attracted to the plant by its color and its copious production of nectar.

Lily of the valley, sometimes written lily-of-the-valley, is a woodland flowering plant with sweetly scented, pendent, bell-shaped white flowers borne in sprays in spring. It is native throughout the cool temperate Northern Hemisphere in Asia and Europe. Convallaria majalis var. montana, also known as the American lily of the valley, is native to North America.

The monarch butterfly or simply monarch is a milkweed butterfly in the family Nymphalidae. Other common names, depending on region, include milkweed, common tiger, wanderer, and black-veined brown. It is amongst the most familiar of North American butterflies and an iconic pollinator, although it is not an especially effective pollinator of milkweeds. Its wings feature an easily recognizable black, orange, and white pattern, with a wingspan of 8.9–10.2 cm (3.5–4.0 in). A Müllerian mimic, the viceroy butterfly, is similar in color and pattern, but is markedly smaller and has an extra black stripe across each hindwing.
Digitoxin is a cardiac glycoside used for the treatment of heart failure and certain kinds of heart arrhythmia. It is a phytosteroid and is similar in structure and effects to digoxin, though the effects are longer-lasting. Unlike digoxin, which is eliminated from the body via the kidneys, it is eliminated via the liver, and so can be used in patients with poor or erratic kidney function. While several controlled trials have shown digoxin to be effective in a proportion of patients treated for heart failure, the evidence base for digitoxin is not as strong, although it is presumed to be similarly effective.

Asclepias is a genus of herbaceous, perennial, flowering plants known as milkweeds, named for their latex, a milky substance containing cardiac glycosides termed cardenolides, exuded where cells are damaged. Most species are toxic to humans and many other species, primarily due to the presence of cardenolides, although, as with many such plants, there are species that feed upon them and from them. Most notable are monarch butterflies, who use and require certain milkweeds as host plants for their larvae.

In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of Heliconius butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body.
Saponins, also selectively referred to as triterpene glycosides, are bitter-tasting usually toxic plant-derived organic chemicals that have a foamy quality when agitated in water. They are widely distributed but found particularly in soapwort, a flowering plant, the soapbark tree and soybeans. They are used in soaps, medicines, fire extinguishers, speciously as dietary supplements, for synthesis of steroids, and in carbonated beverages. Saponins are both water and fat soluble, which gives them their useful soap properties. Some examples of these chemicals are glycyrrhizin and quillaia, a bark extract used in beverages.

Cerberin is a type of cardiac glycoside, a steroidal class found in the seeds of the dicotyledonous angiosperm genus Cerbera; including the suicide tree and the sea mango. This class includes digitalis-like agents, channel-blockers that as a group have found historic uses as cardiac treatments, but which at higher doses are extremely toxic; in the case of cerberin, consumption of the C. odollam results in poisoning with presenting nausea, vomiting, and abdominal pain, often leading to death. The natural product has been structurally characterized, its toxicity is clear—it is often used as an intentional human poison in third-world countries, and accidental poisonings with fatalities have resulted from individuals even indirectly consuming the agent—but its potentially therapeutic pharmacologic properties are very poorly described.

Asclepias curassavica, commonly known as tropical milkweed, is a flowering plant species of the milkweed genus, Asclepias. It is native to the American tropics and has a pantropical distribution as an introduced species. Other common names include bloodflower or blood flower, cotton bush, hierba de la cucaracha, Mexican butterfly weed, redhead, scarlet milkweed, and wild ipecacuanha.

A cardenolide is a type of steroid. Many plants contain derivatives, collectively known as cardenolides, including many in the form of cardenolide glycosides (cardenolides that contain structural groups derived from sugars). Cardenolide glycosides are often toxic; specifically, they are heart-arresting. Cardenolides are toxic to animals through inhibition of the enzyme Na+/K+‐ATPase, which is responsible for maintaining the sodium and potassium ion gradients across the cell membranes.
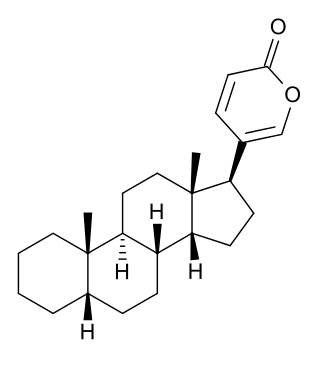
Bufadienolide is a chemical compound with steroid structure. Its derivatives are collectively known as bufadienolides, including many in the form of bufadienolide glycosides. These are a type of cardiac glycoside, the other being the cardenolide glycosides. Both bufadienolides and their glycosides are toxic; specifically, they can cause an atrioventricular block, bradycardia, ventricular tachycardia, and possibly lethal cardiac arrest.

k-Strophanthidin is a cardenolide found in species of the genus Strophanthus. It is the aglycone of k-strophanthin, an analogue of ouabain. k-strophanthin is found in the ripe seeds of Strophanthus kombé and in the lily Convallaria.

Digitalis grandiflora, the yellow foxglove, big-flowered foxglove, or large yellow foxglove, is a species of flowering plant in the genus Digitalis, family Plantaginaceae. It is native to southern Europe and Asia. In mountains it grows on warm, bushy slopes or areas left after logging. The Latin specific epithet grandiflora means “large flowered”.

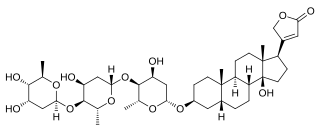
Gitoformate is a cardiac glycoside, a type of drug that can be used in the treatment of congestive heart failure and cardiac arrhythmia. Produced by Madaus, it is not available in the US, and does not seem to be available in Europe either.

Erysimum cheiranthoides, the treacle-mustard,wormseed wallflower, or wormseed mustard is a species of Erysimum native to most of central and northern Europe and northern and central Asia. Like other Erysimum species, E. cheiranthoides accumulates two major classes of defensive chemicals, glucosinolates and cardiac glycosides.
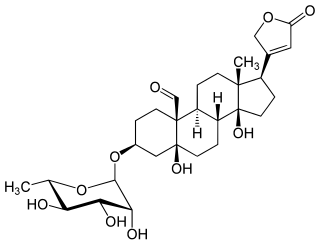
Convallatoxin is a glycoside extracted from Convallaria majalis.

Digitalis ciliata, commonly called hairy foxglove is a member of the genus Digitalis. It has thimble-shaped, yellow to cream colored flowers produced on perennial plants with evergreen foliage. It is native to the Caucasus and is grown as an ornamental in other parts of the world. The species name is derived from the fine hairs that cover the plants stems and flowers.

Erysimum crepidifolium, the pale wallflower, is a plant species in the crucifer family, Brassicaceae. It is a member of the genus Erysimum, which includes between 150 and 350 species in the Northern Hemisphere.



















