The Portuguese man o' war, also known as the man-of-war, is a marine hydrozoan found in the Atlantic Ocean and the Indian Ocean. It is considered to be the same species as the Pacific man o' war or blue bottle, which is found mainly in the Pacific Ocean. The Portuguese man o' war is the only species in the genus Physalia, which in turn is the only genus in the family Physaliidae.

Flavonoids are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans.

Anthocyanidins are common plant pigments, the sugar-free counterparts of anthocyanins. They are based on the flavylium cation, an oxonium ion, with various groups substituted for its hydrogen atoms. They generally change color from red through purple, blue, and bluish green as a function of pH.

Vitis vinifera, the common grape vine, is a species of flowering plant, native to the Mediterranean region, Central Europe, and southwestern Asia, from Morocco and Portugal north to southern Germany and east to northern Iran. There are currently between 5,000 and 10,000 varieties of Vitis vinifera grapes though only a few are of commercial significance for wine and table grape production.

Myrciaria dubia, commonly known as camu-camu, caçari, araçá-d'água, or camocamo, is a species of plant in the family Myrtaceae. It is a small bushy riverside tree from the Amazon rainforest in Peru and Brazil, which grows to a height of 3–5 m (9.8–16.4 ft) and bears a red/purple cherry-like fruit. It is a close relative of the jabuticaba and the guavaberry or rumberry. As much as 2-3% of the fresh fruit by weight is vitamin C.

Physalis is a genus of approximately 75 to 90 flowering plants in the nightshade family (Solanaceae), which are native to the Americas and Australasia. At least 46 species are endemic to Mexico. Cultivated and weedy species have been introduced worldwide. A defining feature of Physalis is a large, papery husk derived from the calyx, which partly or fully encloses the fruit. Many species bear edible fruit, and some species are cultivated.

Apigenin (4′,5,7-trihydroxyflavone), found in many plants, is a natural product belonging to the flavone class that is the aglycone of several naturally occurring glycosides. It is a yellow crystalline solid that has been used to dye wool.

A polyphenol antioxidant is a hypothetical type of antioxidant containing a polyphenolic substructure and studied in vitro. Numbering over 4,000 distinct species mostly from plants, polyphenols may have antioxidant activity in vitro, but are unlikely to be antioxidants in vivo. Hypothetically, they may affect cell-to-cell signaling, receptor sensitivity, inflammatory enzyme activity or gene regulation, although high-quality clinical research has not confirmed any of these possible effects in humans as of 2020.
In enzymology, a dihydrokaempferol 4-reductase (EC 1.1.1.219) is an enzyme that catalyzes the chemical reaction

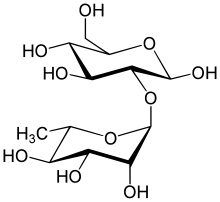
Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time in his treatise "Die Farben der Blüthen". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Rosinidin is an O-methylated anthocyanidin derived from Cyanidin. It is a pigment found in the flowers of Catharanthus roseus and, in lower concentration, in Primula rosea.

Aristotelia chilensis, known as maqui or Chilean wineberry, is a tree species in the Elaeocarpaceae family native to South America in the Valdivian temperate forests of Chile and adjacent regions of southern Argentina. Limited numbers of these trees are cultivated in gardens for their small fruits. Wild-harvested fruits are commercially marketed.

Europinidin (Eu) is an O-methylated anthocyanidin. It is a water-soluble, bluish red plant dye. It is a rare O-methylated flavonoid, a derivative of delphinidin. It can be found in some species of Plumbago and Ceratostigma.
The pyranoanthocyanins are a type of pyranoflavonoids. They are chemical compounds formed in red wines by yeast during fermentation processes or during controlled oxygenation processes during the aging of wine. The different classes of pyranoanthocyanins are carboxypyranoanthocyanins, methylpyranoanthocyanins, pyranoanthocyanin-flavanols, pyranoanthocyanin-phenols, portisins, oxovitisins and pyranoanthocyanin dimers; their general structure includes an additional ring that may have different substituents linked directly at C-10.

Ombuin is an O-methylated flavonol, a type of flavonoid. It is the 4',7-O-methyl derivative of quercetin.

p-Coumaroylated anthocyanins are a type of anthocyanins with a p-coumaric acid unit linked with a sugar to an anthocyanidin aglycone. 3-(6-p-Coumaroyl)glucosides are found in grape and wine. Cyanidin-3-O-(di-p-coumarylglucoside)-5-glucoside is found in dark opal basil. Red leaves of Perilla frutescens also accumulate cyanidin 3-(6-O-p-coumaroyl-β-D-glucoside)-5-(6-O-malonyl-β-D-glucoside).

Ideain, the cyanidin 3-O-galactoside, is an anthocyanin, a type of plant pigment.

Blue flower colour was always associated with something unusual and desired. Blue roses especially were assumed to be a dream that cannot be realised. Blue colour in flower petals is caused by anthocyanins, which are members of flavonoid class metabolites. We can diversify three main classes of anthocyanin pigments: cyaniding type responsible for red coloration, pelargonidin type responsible for orange colour and delphinidin type responsible for violet/blue flower and fruits coloration. The main difference in the structure of listed anthocyanins type is the number of hydroxyl groups in the B-ring of the anthocyanin. Nevertheless, in the monomeric state anthocyanins never show blue colour in the weak acidic and neutral pH. The mechanism of blue colour formation are very complicated in most cases, presence of delphinidin type pigments is not sufficient, great role play also the pH and the formation of complexes of anthocyanins with flavones and metal ions.

Alkekengi officinarum, the bladder cherry, Chinese lantern, Japanese-lantern, strawberry groundcherry, or winter cherry, is a species of flowering plant in the nightshade family Solanaceae. It is a close relative of the new world Calliphysalis carpenteri and a somewhat more distant relative to the members of the Physalis genus. This species is native to the regions covering Southern Europe to South Asia and Northeast Asia.