An antimetabolite is a chemical that inhibits the use of a metabolite, which is another chemical that is part of normal metabolism. Such substances are often similar in structure to the metabolite that they interfere with, such as the antifolates that interfere with the use of folic acid; thus, competitive inhibition can occur, and the presence of antimetabolites can have toxic effects on cells, such as halting cell growth and cell division, so these compounds are used as chemotherapy for cancer.

Fosmidomycin is an antibiotic that was originally isolated from culture broths of bacteria of the genus Streptomyces. It specifically inhibits DXP reductoisomerase, a key enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. It is a structural analogue of 2-C-methyl-D-erythrose 4-phosphate. It inhibits the E. coli enzyme with a KI value of 38 nM (4), MTB at 80 nM, and the Francisella enzyme at 99 nM. Several mutations in the E. coli DXP reductoisomerase were found to confer resistance to fosmidomycin.

Aminoacyl-tRNA is tRNA to which its cognate amino acid is chemically bonded (charged). The aa-tRNA, along with particular elongation factors, deliver the amino acid to the ribosome for incorporation into the polypeptide chain that is being produced during translation.

Rhizoxin is an antimitotic agent with anti-tumor activity. It is isolated from a pathogenic plant fungus which causes rice seedling blight.
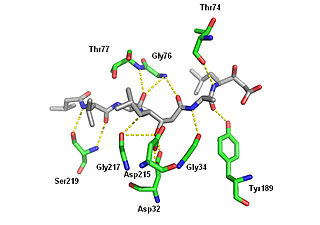
Pepstatin is a potent inhibitor of aspartyl proteases. It is a hexa-peptide containing the unusual amino acid statine, having the sequence Isovaleryl-Val-Val-Sta-Ala-Sta (Iva-Val-Val-Sta-Ala-Sta). It was originally isolated from cultures of various species of Actinomyces due to its ability to inhibit pepsin at picomolar concentrations. Pepstatin A is well known to be an inhibitor of aspartic proteases such as pepsin, cathepsins D and E. Except for its role as a protease inhibitor, however, the pharmacological action of pepstatin A upon cells remain unclear. Pepstatin A suppresses receptor activator of NF-κB ligand (RANKL)–induced osteoclast differentiation. Pepstatin A suppresses the formation of multinuclear osteoclasts dose-dependently. This inhibition of the formation only affected osteoclast cells, i.e., not osteoblast-like cells. Furthermore, pepstatin A also suppresses differentiation from pre-osteoclast cells to mononuclear osteoclast cells dose-dependently. This inhibition seems to be independent of the activities of proteases such as cathepsin D, because the formation of osteoclasts was not suppressed with the concentration that inhibited the activity of cathepsin D. Cell signaling analysis indicated that the phosphorylation of ERK was inhibited in pepstatin A-treated cells, while the phosphorylation of IκB and Akt showed almost no change. Furthermore, pepstatin A decreased the expression of nuclear factor of activated T cells c1 (NFATc1). These results suggest that pepstatin A suppresses the differentiation of osteoclasts through the blockade of ERK signaling and the inhibition of NFATc1 expression.

Aquayamycin is an anthraquinone derivative. It is an inhibitor of the enzyme tyrosine hydroxylase.
A nucleic acid inhibitor is a type of antibacterial that acts by inhibiting the production of nucleic acids. There are two major classes: DNA inhibitors and RNA inhibitors. The antifungal flucytosine acts in a similar manner.

Clavams are a class of antibiotics. This antibiotic is derived from Streptomyces clavuligerus NRRL 3585. Clavam is produced to form a new β-lactam antibiotic. This class is divided into the clavulanic acid class and the 5S clavams class. Clavulanic acid is a broad-spectrum antibiotic and 5S clavams may have anti-fungal properties. They are similar to penams, but with an oxygen substituted for the sulfur. Thus, they are also known as oxapenams.

Kynapcin is a general name for a number of dibenzofuranyl derivatives of the molecule polyozellin, present in the fungus Polyozellus multiplex. Like polyozellin, some kynapcins inhibit prolyl endopeptidase, an enzyme that has a role in processing proteins including amyloid precursor protein. Chemicals that inhibit prolyl endopeptidase have attracted research interest due to their potential therapeutic effects. Several kynapcins have been found in P. multiplex, each with different chemical properties, including kynapcin-12, kynapcin-13 and -28, and -24. A total synthesis of kynapcin-24 was achieved in 2009.
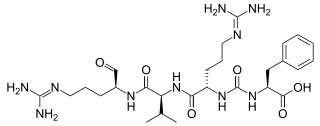
Antipain is an oligopeptide that is isolated from actinomycetes and used in biochemical research as a protease inhibitor of trypsin and papain. It was discovered in 1972 and was the first natural peptide found that contained an ureylene group. Antipain can aid in prevention of coagulation in blood. It is an inhibitor of serine and cysteine proteases.

Statine is a gamma amino acid that occurs twice in the sequence of pepstatin, a protease inhibitor that is active against pepsin and other acid proteases. It is thought to be responsible for the inhibitory activity of pepstatin because it mimics the tetrahedral transition state of peptide catalysis.
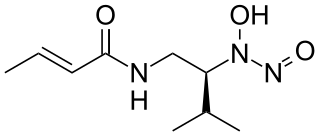
Dopastin is a chemical compound produced by the bacteria Pseudomonas No. BAC-125. It was first isolated and characterized in 1972. It is an inhibitor of the enzyme dopamine β-hydroxylase.
Streptomyces isolates have yielded the majority of human, animal, and agricultural antibiotics, as well as a number of fundamental chemotherapy medicines. Streptomyces is the largest antibiotic-producing genus of Actinomycetota, producing chemotherapy, antibacterial, antifungal, antiparasitic drugs, and immunosuppressants. Streptomyces isolates are typically initiated with the aerial hyphal formation from the mycelium.
Streptomyces amakusaensis is a bacterium species from the genus of Streptomyces which has been isolated from soil from the Amakusa Island in Japan. Streptomyces amakusaensis produces tuberin and nagstatin.
Streptomyces coeruleorubidus is a bacterium species from the genus of Streptomyces which has been isolated from marine sediment. Streptomyces coeruleorubidus produces the following medications: pacidamycin 1, baumycin B1, baumycin B2, baumycin C1, feudomycin A, feudomycin B, feudomycin C, ficellomycin, feudomycinone A, and rubomycin.
Streptomyces griseoaurantiacus is a thermotolerant bacterium species from the genus of Streptomyces which was isolated from marine sediment. Streptomyces griseoaurantiacus produces the antibiotics manumycin, diperamycin and chinikomycin, and griseolic acid.

Bicyclomycin (Bicozamycin) is a broad spectrum antibiotic active against Gram-negative bacteria and the Gram-positive bacterium, Micrococcus luteus that was isolated from Streptomyces sapporonesis and Streptomyces aizumenses in 1972. It belongs to a class of naturally occurring 2,5-diketopiperazines, that are among the most numerous of all the naturally occurring peptide antibiotics. This clinically useful antibiotic is rapidly absorbed in humans when given intramuscularly, has low toxicity and has been used to treat diarrhea in humans and bacterial diarrhea in calves and pigs.

The arylomycins are a class of antibiotics initially isolated from a soil sample obtained in Cape Coast, Ghana. In this initial isolation, two families of closely related arylomycins, A and B, were identified. The family of glycosylated arylomycin C lipopeptides were subsequently isolated from a Streptomyces culture in a screen for inhibitors of bacterial signal peptidase. The initially isolated arylomycins have a limited spectrum of activity against Gram-positive bacteria, including Staphylococcus aureus and Streptococcus pneumoniae. The only activity against Gram-negative bacteria was seen in strains with a compromised outer membrane.

Nagstatin is a strong competitive inhibitor of the N-acetyl-β-d-glucosaminidase with the molecular formula C12H17N3O6. Nagstatin is produced by the bacterium Streptomyces amakusaensis.
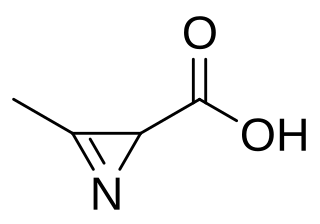
Azirinomycin is an antibiotic azirine derivative with the molecular formula C4H5NO2 which is produced by the bacterium Streptomyces aureus. Azirinomycin was first isolated in 1971. Azirinomycin is toxic and therefore it cannot not be used in human medicine.














