A polymer is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.

Vulcanization is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to include the hardening of other (synthetic) rubbers via various means. Examples include silicone rubber via room temperature vulcanizing and chloroprene rubber (neoprene) using metal oxides.

Wallace Hume Carothers was an American chemist, inventor, and the leader of organic chemistry at DuPont, who was credited with the invention of nylon.

Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle, including during their initial processing, use, disposal into the environment and recycling. The rate of this degradation varies significantly; biodegradation can take decades, whereas some industrial processes can completely decompose a polymer in hours.

An O-ring, also known as a packing or a toric joint, is a mechanical gasket in the shape of a torus; it is a loop of elastomer with a round cross-section, designed to be seated in a groove and compressed during assembly between two or more parts, forming a seal at the interface.
EPDM rubber is a type of synthetic rubber that is used in many applications.

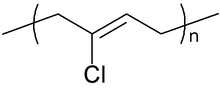
Chloroprene (IUPAC name 2-chlorobuta-1,3-diene) is a chemical compound with the molecular formula CH2=CCl−CH=CH2. Chloroprene is a colorless volatile liquid, almost exclusively used as a monomer for the production of the polymer polychloroprene, better known as neoprene, a type of synthetic rubber.
Elmer Keiser Bolton was an American chemist and research director for DuPont, notable for his role in developing neoprene and directing the research that led to the discovery of nylon.

Julius Aloysius Arthur Nieuwland, CSC, was a Belgian-born Holy Cross priest and professor of chemistry and botany at the University of Notre Dame, Indiana. He is known for his contributions to acetylene research and its use as the basis for one type of synthetic rubber, which eventually led to the invention of neoprene by DuPont.

Embrittlement is a significant decrease of ductility of a material, which makes the material brittle. Embrittlement is used to describe any phenomena where the environment compromises a stressed material's mechanical performance, such as temperature or environmental composition. This is oftentimes undesirable as brittle fracture occurs quicker and can much more easily propagate than ductile fracture, leading to complete failure of the equipment. Various materials have different mechanisms of embrittlement, therefore it can manifest in a variety of ways, from slow crack growth to a reduction of tensile ductility and toughness.

Foam latex or latex foam rubber is a lightweight form of latex containing bubbles known as cells, created from liquid latex. The foam is generally created though the Dunlop or Talalay process in which a liquid latex is foamed and then cured in a mold to extract the foam.
A blowing agent is a substance which is capable of producing a cellular structure via a foaming process in a variety of materials that undergo hardening or phase transition, such as polymers, plastics, and metals. They are typically applied when the blown material is in a liquid stage. The cellular structure in a matrix reduces density, increasing thermal and acoustic insulation, while increasing relative stiffness of the original polymer.

Filler materials are particles added to resin or binders that can improve specific properties, make the product cheaper, or a mixture of both. The two largest segments for filler material use is elastomers and plastics. Worldwide, more than 53 million tons of fillers are used every year in application areas such as paper, plastics, rubber, paints, coatings, adhesives, and sealants. As such, fillers, produced by more than 700 companies, rank among the world's major raw materials and are contained in a variety of goods for daily consumer needs. The top filler materials used are ground calcium carbonate (GCC), precipitated calcium carbonate (PCC), kaolin, talc, and carbon black. Filler materials can affect the tensile strength, toughness, heat resistance, color, clarity, etc. A good example of this is the addition of talc to polypropylene. Most of the filler materials used in plastics are mineral or glass based filler materials. Particulates and fibers are the main subgroups of filler materials. Particulates are small particles of filler that are mixed in the matrix where size and aspect ratio are important. Fibers are small circular strands that can be very long and have very high aspect ratios.
Polymer engineering is generally an engineering field that designs, analyses, and modifies polymer materials. Polymer engineering covers aspects of the petrochemical industry, polymerization, structure and characterization of polymers, properties of polymers, compounding and processing of polymers and description of major polymers, structure property relations and applications.
Electron-beam processing or electron irradiation (EBI) is a process that involves using electrons, usually of high energy, to treat an object for a variety of purposes. This may take place under elevated temperatures and nitrogen atmosphere. Possible uses for electron irradiation include sterilization, alteration of gemstone colors, and cross-linking of polymers.

In polymer chemistry photo-oxidation is the degradation of a polymer surface due to the combined action of light and oxygen. It is the most significant factor in the weathering of plastics. Photo-oxidation causes the polymer chains to break, resulting in the material becoming increasingly brittle. This leads to mechanical failure and, at an advanced stage, the formation of microplastics. In textiles the process is called phototendering.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle.
Ira Williams (1894–1977) was an American chemist at DuPont's Jackson Laboratory in New Jersey, who in the summer of 1930, together with Wallace Carothers, Arnold Collins and F. B. Downing, made commercial Neoprene possible by producing a soft, plastic form of chloroprene that could be processed by the rubber industry. Early accounts of the development credited Julius Nieuwland with synthesizing the precursor divinylacetylene. Williams' contribution was the discovery that the rheological behavior of the product could be controlled by quenching the polymerization reaction with alcohol.
Gérard Berchet was a French-American chemist who played a pivotal role in the invention of both nylon and neoprene. Berchet worked under the direction of Wallace Carothers at DuPont Experimental Station and first synthesized nylon 6 on February 28, 1935, from equal parts hexamethylenediamine and adipic acid. Berchet was the first to synthesize neoprene. However, Arthur Collins is credited with its discovery on April 17, 1930, after he accidentally reacted hydrochloric acid with vinylacetylene. Berchet's leaving of his sample unexamined on a laboratory bench until after Collin's discovery prevented him from being credited with its discovery.
Arnold Miller Collins (1899-1982) was a chemist at DuPont who, working under Elmer Bolton and Wallace Carothers with Ira Williams, first isolated polychloroprene and 2-chloro-1, 3-butadiene in 1930.