
Fusarium is a large genus of filamentous fungi, part of a group often referred to as hyphomycetes, widely distributed in soil and associated with plants. Most species are harmless saprobes, and are relatively abundant members of the soil microbial community. Some species produce mycotoxins in cereal crops that can affect human and animal health if they enter the food chain. The main toxins produced by these Fusarium species are fumonisins and trichothecenes. Despite most species apparently being harmless, some Fusarium species and subspecific groups are among the most important fungal pathogens of plants and animals.

Onychomycosis, also known as tinea unguium, is a fungal infection of the nail. Symptoms may include white or yellow nail discoloration, thickening of the nail, and separation of the nail from the nail bed. Fingernails may be affected, but it is more common for toenails. Complications may include cellulitis of the lower leg. A number of different types of fungus can cause onychomycosis, including dermatophytes and Fusarium. Risk factors include athlete's foot, other nail diseases, exposure to someone with the condition, peripheral vascular disease, and poor immune function. The diagnosis is generally suspected based on the appearance and confirmed by laboratory testing.

Basidiobolus ranarum is a filamentous fungus with worldwide distribution. The fungus was first isolated by Eidam in 1886. It can saprophytically live in the intestines of mainly cold-blooded vertebrates and on decaying fruits and soil. The fungus prefers glucose as a carbon source and grows rapidly at room temperature. Basidiobolus ranarum is also known as a cause of subcutaneous zygomycosis, usually causing granulomatous infections on a host's limbs. Infections are generally geographically limited to tropical and subtropical regions such as East and West Africa. Subcutaneous zygomycosis caused by B. ranarum is a rare disease and predominantly affects children and males. Common subcutaneous zygomycosis shows characteristic features and is relatively easy to be diagnosed; while, certain rare cases might show non-specific clinical features that might pose a difficulty on its identification. Although disease caused by this fungus is known to resolve spontaneously on its own, there are a number of treatments available.

Trichophyton rubrum is a dermatophytic fungus in the phylum Ascomycota. It is an exclusively clonal, anthropophilic saprotroph that colonizes the upper layers of dead skin, and is the most common cause of athlete's foot, fungal infection of nail, jock itch, and ringworm worldwide. Trichophyton rubrum was first described by Malmsten in 1845 and is currently considered to be a complex of species that comprises multiple, geographically patterned morphotypes, several of which have been formally described as distinct taxa, including T. raubitschekii, T. gourvilii, T. megninii and T. soudanense.

Botryosphaeria dothidea is a plant pathogen that causes the formation of cankers on a wide variety of tree and shrub species. It has been reported on several hundred plant hosts and on all continents except Antarctica. B. dothidea was redefined in 2004, and some reports of its host range from prior to that time likely include species that have since been placed in another genus. Even so, B. dothidea has since been identified on a number of woody plants—including grape, mango, olive, eucalyptus, maple, and oak, among others—and is still expected to have a broad geographical distribution. While it is best known as a pathogen, the species has also been identified as an endophyte, existing in association with plant tissues on which disease symptoms were not observed. It can colonize some fruits, in addition to woody tissues.
Exophiala jeanselmei is a saprotrophic fungus in the family Herpotrichiellaceae. Four varieties have been discovered: Exophiala jeanselmei var. heteromorpha, E. jeanselmei var. lecanii-corni, E. jeanselmei var. jeanselmei, and E. jeanselmei var. castellanii. Other species in the genus Exophiala such as E. dermatitidis and E. spinifera have been reported to have similar annellidic conidiogenesis and may therefore be difficult to differentiate.
Pathogenic fungi are fungi that cause disease in humans or other organisms. Although fungi are eukaryotic, many pathogenic fungi are microorganisms. Approximately 300 fungi are known to be pathogenic to humans; their study is called "medical mycology". Fungal infections kill more people than either tuberculosis or malaria—about 2 million people per year.
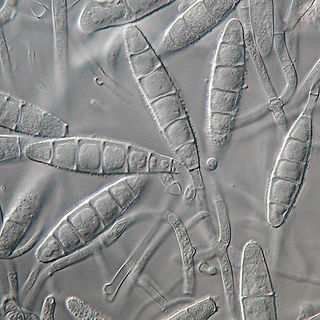
Microsporum gypseum is a soil-associated dermatophyte that occasionally is known to colonise and infect the upper dead layers of the skin of mammals. The name refers to an asexual "form-taxon" that has been associated with four related biological species of fungi: the pathogenic taxa Arthroderma incurvatum, A. gypsea, A. fulva and the non-pathogenic saprotroph A. corniculata. More recent studies have restricted M. gypseum to two teleomorphic species A. gypseum and A. incurvatum. The conidial states of A. fulva and A. corniculata have been assigned to M. fulvum and M. boullardii. Because the anamorphic states of these fungi are so similar, they can be identified reliably only by mating. Two mating strains have been discovered, "+" and "–". The classification of this species has been based on the characteristically rough-walled, blunt, club-shaped, multicelled macroconidia. Synonyms include Achorion gypseum, Microsporum flavescens, M. scorteum, and M. xanthodes. There has been past nomenclatural confusion in the usage of the generic names Microsporum and Microsporon.

Scytalidium is a genus of fungi in the Helotiales order. The relationship of this taxon to other taxa within the order is unknown, and it has not yet been placed with certainty into any family. This genus of anamorphic fungi has a widespread distribution and contains 18 species. Scytalidium dimidiatum causes onychomycosis in tea leaf pluckers.

Cladophialophora bantiana is a melanin producing mold known to cause brain abscesses in humans. It is one of the most common causes of systemic phaeohyphomycosis in mammals. Cladophialophora bantiana is a member of the ascomycota and has been isolated from soil samples from around the world.

Onychocola canadensis is a relative of the dermatophyte and an occasionally causes onychomycosis. It was described in 1990 from 3 clinical reports in Canada.
Coniochaeta hoffmannii, also known as Lecythophora hoffmannii, is an ascomycete fungus that grows commonly in soil. It has also been categorized as a soft-rot fungus capable of bringing the surface layer of timber into a state of decay, even when safeguarded with preservatives. Additionally, it has pathogenic properties, although it causes serious infection only in rare cases. A plant pathogen lacking a known sexual state, C. hoffmannii has been classified as a "dematiaceous fungus" despite its contradictory lack of pigmentation; both in vivo and in vitro, there is no correlation between its appearance and its classification.
Lasiodiplodia citricola is an endophytic fungus. It was first isolated in northern Iran, and is named after its first known host, citrus plants. It has since been isolated in other plants in other continents, and is considered a plant pathogen. L. citricola is phylogenetically related to L. parva, but conidia of the former are longer and wider.

Rhinocladiella mackenziei is a deeply pigmented mold that is a common cause of human cerebral phaeohyphomycosis. Rhinocladiella mackenziei was believed to be endemic solely to the Middle East, due to the first cases of infection being limited to the region. However, cases of R. mackenziei infection are increasingly reported from regions outside the Middle East. This pathogen is unique in that the majority of cases have been reported from immunologically normal people.
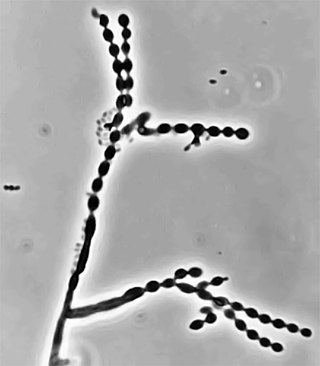
Cladophialophora carrionii is a melanized fungus in the genus Cladophialophora that is associated with decaying plant material like cacti and wood. It is one of the most frequent species of Cladophialophora implicated in human disease. Cladophialophora carrionii is a causative agent of chromoblastomycosis, a subcutaneous infection that occurs in sub-tropical areas such as Madagascar, Australia and northwestern Venezuela. Transmission occurs through traumatic implantation of plant material colonized by C. carrionii, mainly infecting rural workers. When C. carrionii infects its host, it transforms from a mycelial state to a muriform state to better tolerate the extreme conditions in the host's body.

Epidermophyton floccosum is a filamentous fungus that causes skin and nail infections in humans. This anthropophilic dermatophyte can lead to diseases such as tinea pedis, tinea cruris, tinea corporis and onychomycosis. Diagnostic approaches of the fungal infection include physical examination, culture testing, and molecular detection. Topical antifungal treatment, such as the use of terbinafine, itraconazole, voriconazole, and ketoconazole, is often effective.
Sarocladium kiliense is a saprobic fungus that is occasionally encountered as a opportunistic pathogen of humans, particularly immunocompromised and individuals. The fungus is frequently found in soil and has been linked with skin and systemic infections. This species is also known to cause disease in the green alga, Cladophora glomerata as well as various fruit and vegetable crops grown in warmer climates.
Arthrographis kalrae is an ascomycetous fungus responsible for human nail infections described in 1938 by Cochet as A. langeronii. A. kalrae is considered a weak pathogen of animals including human restricted to the outermost keratinized layers of tissue. Infections caused by this species are normally responsive to commonly used antifungal drugs with only very rare exceptions.
Neofusicoccum arbuti is a fungus species in the genus Neofusicoccum. It was first described by D.F. Farr & M. Elliott, and given its current name by Crous, Slippers & A.J.L. Phillips in 2006. Neofusicoccum arbuti is included in the genus Neofusicoccum and the family Botryosphaeriaceae. This species is known as madrone canker. N. arbuti is a potentially lethal canker disease of Pacific madrone, Arbutus menziesii.
Scytalidium hyalinum is an ascomycete fungus currently in the genus Scytalidium. It causes dermatomycosis and systemic infections in humans and it is widespread throughout the world.













