Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.

Nymphaea is a genus of hardy and tender aquatic plants in the family Nymphaeaceae. The genus has a cosmopolitan distribution. Many species are cultivated as ornamental plants, and many cultivars have been bred. Some taxa occur as introduced species where they are not native, and some are weeds. Plants of the genus are known commonly as water lilies, or waterlilies in the United Kingdom. The genus name is from the Greek νυμφαία, nymphaia and the Latin nymphaea, which mean "water lily" and were inspired by the nymphs of Greek and Latin mythology.

Vinca is a genus of flowering plants in the family Apocynaceae, native to Europe, northwest Africa and southwest Asia. The English name periwinkle is shared with the related genus Catharanthus.

Nuphar is a genus of aquatic plants in the family Nymphaeaceae, with a temperate to subarctic Northern Hemisphere distribution. Common names include water-lily, pond-lily, alligator-bonnet or bonnet lily, and spatterdock.
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. The molecule consists of a five-membered saturated ring with four methylene groups and a sulfur atom. It is the saturated analog of thiophene. It is a volatile, colorless liquid with an intensely unpleasant odor. It is also known as thiophane, thiolane, or THT. While THT is not particularly common, the vitamin biotin is essential for life in aerobic organisms.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

Nuphar lutea, the yellow water-lily, brandy-bottle, or spadderdock, is an aquatic plant of the family Nymphaeaceae, native to northern temperate and some subtropical regions of Europe, northwest Africa, western Asia, North America, and Cuba. This interesting species found on both sides of the Atlantic Ocean was used as a food source and in medicinal practices from prehistoric times with potential research and medical applications going forward.

Osman Achmatowicz was a Polish professor of chemistry of Lipka Tatar descent. His son, Osman Achmatowicz Jr., is credited with the Achmatowicz reaction in 1971.
The molecular formula C15H25NO2 may refer to:

Palau'amine is a toxic alkaloid compound synthesized naturally by Stylotella agminata, a species of sea sponge found in the southwest Pacific Ocean. The name of the molecule derives from the island nation of Palau, near which the sponges are found.

Nuphar pumila, the least water-lily or small yellow pond-lily, is an aquatic perennial plant in the Nymphaeaceae family. It is also known as the dwarf water lily since it looks like a smaller Nuphar lutea. while Nuphar pumila has a star-shaped, or lobed form of the stigma disc and glabrous leaf undersides, Nuphar lutea has a round stigma disc and the undersides of its leaves are occasionally fine-haired on the midribs. Its flowers bloom from July to August and are typically pollinated by flies.

Voacamine, also known under the older names voacanginine and vocamine, is a naturally occurring dimeric indole alkaloid of the secologanin type, found in a number of plants, including Voacanga africana and Tabernaemontana divaricata. It is approved for use as an antimalarial drug in several African countries. Voacamine exhibits cannabinoid CB1 receptor antagonistic activity.

Biphalin is a dimeric enkephalin endogenous peptide (Tyr-D-Ala-Gly-Phe-NH)2 composed of two tetrapeptides derived from enkephalins, connected 'tail-to-tail' by a hydrazide bridge. The presence of two distinct pharmacophores confers on biphalin a high affinity for both μ and δ opioid receptors (with an EC50 of about 1-5 nM for both μ and δ receptors), therefore it has analgesic activity. Biphalin presents a considerable antinociceptive profile. In fact, when administered intracerebroventricularly in mice, biphalin displays a potency almost 7-fold greater than that of the ultra-potent alkaloid agonist, etorphine and 7000-fold greater than morphine; biphalin and morphine were found to be equipotent after intraperitoneal administration. The extraordinary in vivo potency shown by this compound is coupled with low side-effects, in particular, to produce no dependency in chronic use. For these reasons, several efforts have been carried out in order to obtain more information about structure-activity relationship (SAR). Results clearly indicate that, at least for μ receptor binding, the presence of two pharmacophores is not necessary; Tyr1 is indispensable for analgesic activity, while replacing Phe at the position 4 and 4' with non-aromatic, but lipophilic amino acids does not greatly change the binding properties and in general 4,4' positions are found to be important to design biphalin analogues with increased potency and modified μ/δ selectivity. The hydrazide linker is not fundamental for activity or binding, and it can be conveniently substituted by different conformationally constrained cycloaliphatic diamine linkers.

Nuphar japonica, known as East Asian yellow water-lily, is an aquatic plant species in the genus Nuphar found in Japan and the Korean Peninsula. It is endangered in Russia. The species was not accepted by The Plant List as of November 2013, which regarded it as an "unresolved name".
The molecular formula C26H26N2 (molar mass: 366.49 g/mol) may refer to:

Strychnos usambarensis is a shrub or small tree up to 15m tall or a 70m long liane of Sub-Saharan Africa, occurring in forest and woodland, mountain ravines and coastal bush, often on rocky slopes and named for the Usambara Mountains of Tanzania. The species is found from Guinea east to Nigeria, from Congo east to Kenya and south to kloofs of the Magaliesberg of South Africa. Bantu tribes from Rwanda and Tanzania produce an arrow poison from the root bark and leaves of this species, sometimes combining it with extracts from other plants.

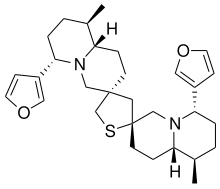
Nupharamine is an alkaloid found in Nuphar japonica and in castoreum.

Isovoacangine is a naturally occurring substance that has action on heart muscles in pigs

Conophylline is a autophagy inducing vinca alkaloid found in several species of Tabernaemontana including Ervatamia microphylla and Tabernaemontana divaricata. Among its many functional groups is an epoxide: the compound where that ring is replaced with a double bond is called conophyllidine and this co-occurs in the same plants.