
Ascaris lumbricoides is a large parasitic worm that causes ascariasis in humans. A roundworm of genus Ascaris, it is the most common parasitic worm in humans. An estimated 807 million–1.2 billion people are infected with A. lumbricoides worldwide. People living in tropical and subtropical countries are at greater risk of infection.
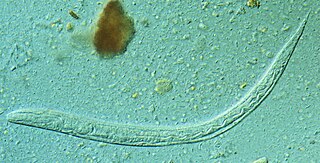
Strongyloides stercoralis is a human pathogenic parasitic roundworm causing the disease strongyloidiasis. Its common name in the US is threadworm. In the UK and Australia, however, the term threadworm can also refer to nematodes of the genus Enterobius, otherwise known as pinworms.
Toxocariasis is an illness of humans caused by the dog roundworm and, less frequently, the cat roundworm. These are the most common intestinal roundworms of dogs, coyotes, wolves and foxes and domestic cats, respectively. Humans are among the many "accidental" or paratenic hosts of these roundworms.
Hymenolepiasis is infestation by one of two species of tapeworm: Hymenolepis nana or H. diminuta. Alternative names are dwarf tapeworm infection and rat tapeworm infection. The disease is a type of helminthiasis which is classified as a neglected tropical disease.

Necator americanus is a species of hookworm commonly known as the New World hookworm. Like other hookworms, it is a member of the phylum Nematoda. It is an obligatory parasitic nematode that lives in the small intestine of human hosts. Necatoriasis—a type of helminthiasis—is the term for the condition of being host to an infestation of a species of Necator. Since N. americanus and Ancylostoma duodenale are the two species of hookworms that most commonly infest humans, they are usually dealt with under the collective heading of "hookworm infection". They differ most obviously in geographical distribution, structure of mouthparts, and relative size.

Trichobilharzia regenti is a neuropathogenic parasitic flatworm of birds which also causes cercarial dermatitis in humans. The species was originally described in 1998 in the Czech Republic and afterwards it was detected also in other European countries, e.g. Denmark, Germany, France, Iceland, Poland, Switzerland, or Russia, and even in Iran. For its unique neurotropic behaviour in vertebrate hosts, the host-parasite interactions are extensively studied in terms of molecular biology, biochemistry and immunology.
Trichuris muris is a nematode parasite of mice. It is very similar to the human roundworm parasite Trichuris trichiura due to its immunological reactivity when crossed, and so is often used in related studies.

Heligmosomoides polygyrus, previously named Nematospiroides dubius, is a naturally occurring intestinal roundworm of rodents. It belongs to the family Trychostrongylidae, and male and female worms are morphologically distinguishable. The parasite has a direct lifecycle, with its larval form being the infective stage. H. polygyrus has the ability to establish chronic infections in rodents and alter host immune responses. This nematode is widely used as a gastrointestinal parasitic model in immunological, pharmacological, and toxicological studies.

Trichuris suis is a whipworm; the variations in thickness of the anterior and posterior segments give the parasite the characteristic "whip-like" appearance. Adult females measure 6 to 8 cm and adult males 3 to 4 cm. T. suis eggs are oval and yellow-brown with bipolar plugs. T. suis is also used in helminthic therapy studies.
Muelleries capillaris, also known as the hair or goat lungworm, is one of the most economically important nematodes of small ruminants. Although normally non-pathogenic in sheep, the parasite causes a disease condition called muelleriosis in goats. Sheep and goats commonly become infected after accidentally ingesting M. capillaris infected snails or slugs, and the parasite produces eggs in the lungs of its host, causing life-threatening bronchopneumonia in serious cases.

Toxascaris leonina is a common parasitic roundworm found in dogs, cats, foxes, and related host species. T. leonina is an ascarid nematode, a worldwide distributed helminth parasite which is in a division of eukaryotic parasites that, unlike external parasites such as lice and fleas, live inside their host. The definitive hosts of T. leonina include canids and felines (cats), while the intermediate hosts are usually rodents, such as mice or rats. Infection occurs in the definitive host when the animal eats an infected rodent. While T. leonina can occur in either dogs or cats, it is far more frequent in cats.

Capillaria hepatica is a parasitic nematode which causes hepatic capillariasis in rodents and numerous other mammal species, including humans. The life cycle of C. hepatica may be completed in a single host species. However, the eggs, which are laid in the liver, must mature outside of the host body prior to infecting a new host. So the death of the host in which the adults reach sexual maturity, either by being eaten or dying and decomposing, is necessary for completion of the life cycle.

Ancylostoma caninum is a species of nematode known as a hookworm, which principally infects the small intestine of dogs. The result of A. caninum infection ranges from asymptomatic cases to death of the dog; better nourishment, increasing age, prior A. caninum exposure, or vaccination are all linked to improved survival. Other hosts include carnivores such as wolves, foxes, and cats, with a small number of cases having been reported in humans.
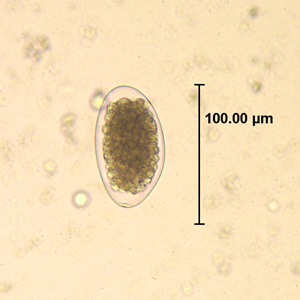
Trichostrongylus species are nematodes, which are ubiquitous among herbivores worldwide, including cattle, sheep, donkeys, goats, deer, and rabbits. At least 10 Trichostrongylus species have been associated with human infections. Infections occur via ingestion of infective larvae from contaminated vegetables or water. Epidemiological studies indicate a worldwide distribution of Trichostrongylus infections in humans, with the highest prevalence rates observed in individuals from regions with poor sanitary conditions, in rural areas, or who are farmers / herders. Human infections are most prevalent in the Middle East and Asia, with a worldwide estimated prevalence of 5.5 million people.

Angiostrongylus vasorum, also known as French heartworm, is a species of parasitic nematode in the family Metastrongylidae. It causes the disease canine angiostrongylosis in dogs. It is not zoonotic, that is, it cannot be transmitted to humans.
The effects of parasitic worms, or helminths, on the immune system is a recently emerging topic of study among immunologists and other biologists. Experiments have involved a wide range of parasites, diseases, and hosts. The effects on humans have been of special interest. The tendency of many parasitic worms to pacify the host's immune response allows them to mollify some diseases, while worsening others.

Teladorsagia circumcincta is a nematode that is one of the most important parasites of sheep and goats. It was previously known as Ostertagia circumcincta and is colloquially known as the brown stomach worm. It is common in cool, temperate areas, such as south-eastern and south-western Australia and the United Kingdom. There is considerable variation among lambs and kids in susceptibility to infection. Much of the variation is genetic and influences the immune response. The parasite induces a type I hypersensitivity response which is responsible for the relative protein deficiency which is characteristic of severely infected animals. There are mechanistic mathematical models which can predict the course of infection. There are a variety of ways to control the infection and a combination of control measures is likely to provide the most effective and sustainable control.
Eustrongylidosis is a parasitic disease that mainly affects wading birds worldwide; however, the parasite's complex, indirect lifecycle involves other species, such as aquatic worms and fish. Moreover, this disease is zoonotic, which means the parasite can transmit disease from animals to humans. Eustrongylidosis is named after the causative agent Eustrongylides, and typically occurs in eutrophicated waters where concentrations of nutrients and minerals are high enough to provide ideal conditions for the parasite to thrive and persist. Because eutrophication has become a common issue due to agricultural runoff and urban development, cases of eustrongylidosis are becoming prevalent and hard to control. Eustrongylidosis can be diagnosed before or after death by observing behavior and clinical signs, and performing fecal flotations and necropsies. Methods to control it include preventing eutrophication and providing hosts with uninfected food sources in aquaculture farms. Parasites are known to be indicators of environmental health and stability, so should be studied further to better understand the parasite's lifecycle and how it affects predator-prey interactions and improve conservation efforts.

Parascaris equorum is a species of ascarid that is the equine roundworm. Amongst horse owners, the parasites are colloquially called "Ascarids". This is a host-specific helminth intestinal parasite that can infect horses, donkeys, and zebras. Horses up to six months of age are the most susceptible to infection. After this time, infection rates begin to decline and is extremely uncommon in horses over twelve months of age. It cannot infect humans or other animals. It is yellow-white in color, and females can become as large as 15 inches (38 cm) in length. Found worldwide, P. equorum is one of the most difficult equine parasites to kill, requiring larger doses of more powerful anthelmintic medications than are needed for other equine parasites.

Hookworms are intestinal, blood-feeding, parasitic roundworms that cause types of infection known as helminthiases. Hookworm infection is found in many parts of the world, and is common in areas with poor access to adequate water, sanitation, and hygiene. In humans, infections are caused by two main species of roundworm, belonging to the genera Ancylostoma and Necator. In other animals the main parasites are species of Ancylostoma. Hookworm is closely associated with poverty because it is most often found in impoverished areas, and its symptoms promote poverty through the educational and health effects it has on children. It is the leading cause of anemia and undernutrition in developing countries, while being one of the most commonly occurring diseases among poor people. Hookworm thrives in areas where rainfall is sufficient and keeps the soil from drying out, and where temperatures are higher, making rural, coastal areas prime conditions for the parasite to breed.














