Urea, also called carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two amino groups joined by a carbonyl functional group. It is thus the simplest amide of carbamic acid.

A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment or hand-tool methods.

Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.
A prill is a small aggregate or globule of a material, most often a dry sphere, formed from a melted liquid through spray crystallization. Prilled is a term used in mining and manufacturing to refer to a product that has been pelletized. ANFO explosive typically comprises ammonium nitrate prills mixed with #2 fuel oil. The pellets are a neater, simpler form for handling, with reduced dust.
UAN is a solution of urea and ammonium nitrate in water used as a fertilizer. The combination of urea and ammonium nitrate has an extremely low critical relative humidity and can therefore only be used in liquid fertilizers. The most commonly used grade of these fertilizer solutions is UAN 32.0.0 (32%N) known as UN32 or UN-32, which consists of 45% ammonium nitrate, 35% urea and only 20% water. Other grades are UAN 28, UAN 30 and UAN 18. The solutions are quite corrosive towards mild steel and are therefore generally equipped with a corrosion inhibitor to protect tanks, pipelines, nozzles, etc. Urea–ammonium nitrate solutions should not be combined with calcium ammonium nitrate (CAN-17) or other solutions prepared from calcium nitrate. A thick, milky-white insoluble precipitate forms that may plug nozzles.

Calcium nitrate are inorganic compounds with the formula Ca(NO3)2(H2O)x. The anhydrous compound, which is rarely encountered, absorbs moisture from the air to give the tetrahydrate. Both anhydrous and hydrated forms are colourless salts. Hydrated calcium nitrate, also called Norgessalpeter (Norwegian salpeter), is mainly used as a component in fertilizers, but it has other applications. Nitrocalcite is the name for a mineral which is a hydrated calcium nitrate that forms as an efflorescence where manure contacts concrete or limestone in a dry environment as in stables or caverns. A variety of related salts are known including calcium ammonium nitrate decahydrate and calcium potassium nitrate decahydrate.
The Berlin Method of biological filtration is a method for maintaining a clean and stable environment within a saltwater aquarium, typically a coral reef system. This method relies on the use of ample live rock. The theory is that aerobic bacteria covering the surface of the porous live rock and sand convert harmful ammonia into nitrites, then nitrates, which are much less harmful to the tank's inhabitants. Through the process of diffusion, the nitrates move deep within the rock where they are converted by anaerobic bacteria to free nitrogen gas. Left over nitrates are removed through regular partial water changes, or with algal filtration such as an algae scrubber. As an added measure, a protein skimmer is used to remove some of the dissolved organic compounds before they break down into ammonia, although skimmers do not remove ammonia from fish urea.

Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, sulphamic acid and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colourless, water-soluble compound finds many applications. Sulfamic acid melts at 205 °C before decomposing at higher temperatures to water, sulfur trioxide, sulfur dioxide and nitrogen.

Verdigris is an incorporated town in Rogers County, Oklahoma, United States, in the Tulsa metropolitan area. It straddles former U.S. Highway 66 between Catoosa and Claremore. Verdigris had a population of 3,993 at the 2010 census, an increase of 92.9 percent from 2,070 at the 2000 census.
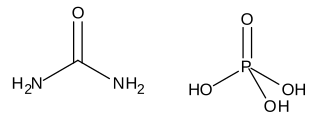
Urea phosphate is a 1:1 combination of urea and phosphoric acid that is used as a fertilizer. It has an NPK formula 17-44-0, and is soluble in water, producing a strongly acidic solution.
Metabolic wastes or excrements are substances left over from metabolic processes (such as cellular respiration) which cannot be used by the organism (they are surplus or toxic), and must therefore be excreted. This includes nitrogen compounds, water, CO2, phosphates, sulphates, etc. Animals treat these compounds as excretes. Plants have metabolic pathways which transforms some of them (primarily the oxygen compounds) into useful substances.

National Fertilizers Limited (NFL) is an Indian central public sector undertaking and the largest government-owned-Urea fertilizer-producer in India. It is a Navratna company, with the Government of India owning a majority stake.
The critical relative humidity (CRH) of a salt is defined as the relative humidity of the surrounding atmosphere at which the material begins to absorb moisture from the atmosphere and below which it will not absorb atmospheric moisture.

The Wolffenstein–Böters reaction is an organic reaction converting benzene to picric acid by a mixture of aqueous nitric acid and mercury(II) nitrate.
Berthelot's reagent is an alkaline solution of phenol and hypochlorite, used in analytical chemistry. It is named after its inventor, Marcellin Berthelot. Ammonia reacts with Berthelot's reagent to form a blue product which is used in a colorimetric method for determining ammonia. The reagent can also be used for determining urea. In this case the enzyme urease is used to catalyze the hydrolysis of urea into carbon dioxide and ammonia. The ammonia is then determined with Berthelot's reagent.

Urea nitrate is a fertilizer-based high explosive that has been used in improvised explosive devices in Afghanistan, Pakistan, Iraq, and various terrorist acts elsewhere in the world such as in the 1993 World Trade Center bombings. It has a destructive power similar to better-known ammonium nitrate explosives, with a velocity of detonation between 3,400 m/s (11,155 ft/s) and 4,700 m/s (15,420 ft/s). It has chemical formula of CH5N3O4 or (NH2)2COHNO3.

Yara Belle Plaine Inc is a nitrate-based fertilizer manufacturer based in Regina, Saskatchewan, Canada, and is part of the Yara International group of companies.

Grupa Azoty Zakłady Azotowe "Puławy" PLC is a Polish chemical company based in Puławy, Lublin Voivodeship, specializing in the production of mono-volumes of nitrogen fertilizer, one of the world's largest producers of melamine. It also produces caprolactam, hydrogen peroxide, AdBlue and technical gases.
Stamicarbon is the licensing and IP center of Maire Tecnimont SpA which licenses technology for manufacturing urea as well as provide follow-up services designed to ensure the best possible operation of the urea plant throughout its working life. Stamicarbon is based in Sittard-Geleen.
Ostchem Holding is a holding company that unites a group of chemical factories and supporting companies. In its turn Ostchem is a part of bigger Group DF that unites several separate enterprises and other holding companies and is owned by Ukrainian oligarch Dmytro Firtash.











