
The noble gases are the naturally occurring members of group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Under standard conditions, these elements are odorless, colorless, monatomic gases with very low chemical reactivity and cryogenic boiling points.
Xenon tetroxide is a chemical compound of xenon and oxygen with molecular formula XeO4, remarkable for being a relatively stable compound of a noble gas. It is a yellow crystalline solid that is stable below −35.9 °C; above that temperature it is very prone to exploding and decomposing into elemental xenon and oxygen (O2).
Elastic properties describe the reversible deformation of a material to an applied stress. They are a subset of the material properties that provide a quantitative description of the characteristics of a material, like its strength.
The speed of sound in any chemical element in the fluid phase has one temperature-dependent value. In the solid phase, different types of sound wave may be propagated, each with its own speed: among these types of wave are longitudinal, transversal, and extensional.
This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus as an example, the concise form is [Ne] 3s2 3p3. Here [Ne] refers to the core electrons which are the same as for the element neon (Ne), the last noble gas before phosphorus in the periodic table. The valence electrons are written explicitly for all atoms.

Tungsten oxytetrafluoride is an inorganic compound with the formula WOF4. It is a colorless diamagnetic solid. The compound is one of many oxides of tungsten. It is usually encountered as product of the partial hydrolysis of tungsten hexafluoride.
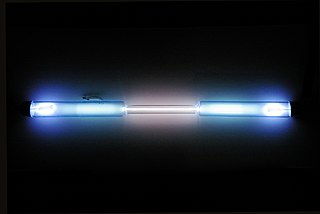
Krypton is a chemical element; it has symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is often used with other rare gases in fluorescent lamps. Krypton is chemically inert.
A hexafluoride is a chemical compound with the general formula QXnF6, QXnF6m−, or QXnF6m+. Many molecules fit this formula. An important hexafluoride is hexafluorosilicic acid (H2SiF6), which is a byproduct of the mining of phosphate rock. In the nuclear industry, uranium hexafluoride (UF6) is an important intermediate in the purification of this element.

Bromine dioxide is the chemical compound composed of bromine and oxygen with the formula BrO2. It forms unstable yellow to yellow-orange crystals. It was first isolated by R. Schwarz and M. Schmeißer in 1937 and is hypothesized to be important in the atmospheric reaction of bromine with ozone. It is similar to chlorine dioxide, the dioxide of its halogen neighbor one period higher on the periodic table.

2-Pentanol is an organic chemical compound. It is used as a solvent and an intermediate in the manufacturing of other chemicals. 2-Pentanol is a component of many mixtures of amyl alcohols sold industrially. 2-Pentanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(−)-2-pentanol and (S)-(+)-2-pentanol.
Silicon monosulfide is a chemical compound of silicon and sulfur. The chemical formula is SiS. Molecular SiS has been detected at high temperature in the gas phase. The gas phase molecule has an Si-S bondlength of 192.93 pm, this compares to the normal single bond length of 216 pm, and is shorter than the Si=S bond length of around 201 pm reported in an organosilanethione. Historically a pale yellow-red amorphous solid compound has been reported. The behavior of silicon can be contrasted with germanium which forms a stable solid monosulfide.

Radon compounds are chemical compounds formed by the element radon (Rn). Radon is a noble gas, i.e. a zero-valence element, and is chemically not very reactive. The 3.8-day half-life of radon-222 makes it useful in physical sciences as a natural tracer. Because radon is a gas under normal circumstances, and its decay-chain parents are not, it can readily be extracted from them for research.