Related Research Articles
Background radiation is a measure of the level of ionizing radiation present in the environment at a particular location which is not due to deliberate introduction of radiation sources.
A radionuclide is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (t1/2) for that collection, can be calculated from their measured decay constants. The range of the half-lives of radioactive atoms has no known limits and spans a time range of over 55 orders of magnitude.
Nuclear fallout is the residual radioactive material propelled into the upper atmosphere following a nuclear blast, so called because it "falls out" of the sky after the explosion and the shock wave has passed. It commonly refers to the radioactive dust and ash created when a nuclear weapon explodes. The amount and spread of fallout is a product of the size of the weapon and the altitude at which it is detonated. Fallout may get entrained with the products of a pyrocumulus cloud and fall as black rain. This radioactive dust, usually consisting of fission products mixed with bystanding atoms that are neutron-activated by exposure, is a form of radioactive contamination.

A mushroom cloud is a distinctive mushroom-shaped flammagenitus cloud of debris, smoke and usually condensed water vapor resulting from a large explosion. The effect is most commonly associated with a nuclear explosion, but any sufficiently energetic detonation or deflagration will produce the same effect. They can be caused by powerful conventional weapons, like thermobaric weapons, including the ATBIP and GBU-43/B Massive Ordnance Air Blast. Some volcanic eruptions and impact events can produce natural mushroom clouds.

A nuclear and radiation accident is defined by the International Atomic Energy Agency (IAEA) as "an event that has led to significant consequences to people, the environment or the facility. Examples include lethal effects to individuals, large radioactivity release to the environment, reactor core melt." The prime example of a "major nuclear accident" is one in which a reactor core is damaged and significant amounts of radioactive isotopes are released, such as in the Chernobyl disaster in 1986 and Fukushima nuclear disaster in 2011.

Radioactive contamination, also called radiological pollution, is the deposition of, or presence of radioactive substances on surfaces or within solids, liquids, or gases, where their presence is unintended or undesirable.

Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy, and gamma rays. The two smaller nuclei are the fission products..
Polonium-210 is an isotope of polonium. It undergoes alpha decay to stable 206Pb with a half-life of 138.376 days, the longest half-life of all naturally occurring polonium isotopes. First identified in 1898, and also marking the discovery of the element polonium, 210Po is generated in the decay chain of uranium-238 and radium-226. 210Po is a prominent contaminant in the environment, mostly affecting seafood and tobacco. Its extreme toxicity is attributed to intense radioactivity, capable of severely harming humans.
The Mayak Production Association is one of the biggest nuclear facilities in the Russian Federation, housing a reprocessing plant. The closest settlements are Ozyorsk to the northwest and Novogornyi to the south.

The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two nuclear energy accidents rated at seven—the maximum severity—on the International Nuclear Event Scale, the other being the 2011 Fukushima nuclear disaster in Japan. The initial emergency response, together with later decontamination of the environment, involved more than 500,000 personnel and cost an estimated 18 billion roubles—roughly US$68 billion in 2019, adjusted for inflation.

A hot particle is a microscopic piece of radioactive material that can become lodged in living tissue and deliver a concentrated dose of radiation to a small area. A controversial theory proposes that hot particles within the body are vastly more dangerous than external emitters delivering the same dose of radiation in a diffused manner. Other researchers claim that there is little or no difference in risk between internal and external emitters. Individuals will likely continue to accumulate radiation dose from internal sources even after being removed from the original hazard and properly decontaminated, regardless of the relative danger from an internally sourced radiation dose compared to an equivalent externally sourced radiation dose.
Radionuclides which emit gamma radiation are valuable in a range of different industrial, scientific and medical technologies. This article lists some common gamma-emitting radionuclides of technological importance, and their properties.
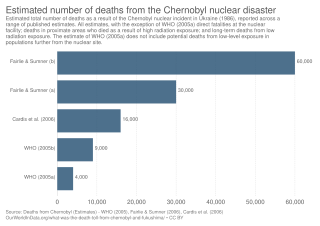
The 1986 Chernobyl disaster triggered the release of radioactive contamination into the atmosphere in the form of both particulate and gaseous radioisotopes. As of 2022, it was the world's largest known release of radioactivity into the environment.

This page discusses each of the main elements in the mixture of fission products produced by nuclear fission of the common nuclear fuels uranium and plutonium. The isotopes are listed by element, in order by atomic number.

Environmental radioactivity is produced by radioactive materials in the human environment. While some radioisotopes, such as strontium-90 (90Sr) and technetium-99 (99Tc), are only found on Earth as a result of human activity, and some, like potassium-40 (40K), are only present due to natural processes, a few isotopes, e.g. tritium (3H), result from both natural processes and human activities. The concentration and location of some natural isotopes, particularly uranium-238 (238U), can be affected by human activity.

Strontium-90 is a radioactive isotope of strontium produced by nuclear fission, with a half-life of 28.8 years. It undergoes β− decay into yttrium-90, with a decay energy of 0.546 MeV. Strontium-90 has applications in medicine and industry and is an isotope of concern in fallout from nuclear weapons, nuclear weapons testing, and nuclear accidents.
Naturally occurring radioactive materials (NORM) and technologically enhanced naturally occurring radioactive materials (TENORM) consist of materials, usually industrial wastes or by-products enriched with radioactive elements found in the environment, such as uranium, thorium and potassium and any of their decay products, such as radium and radon. Produced water discharges and spills are a good example of entering NORMs into the surrounding environment.

Radioecology is the branch of ecology concerning the presence of radioactivity in Earth’s ecosystems. Investigations in radioecology include field sampling, experimental field and laboratory procedures, and the development of environmentally predictive simulation models in an attempt to understand the migration methods of radioactive material throughout the environment.

Bioremediation of radioactive waste or bioremediation of radionuclides is an application of bioremediation based on the use of biological agents bacteria, plants and fungi to catalyze chemical reactions that allow the decontamination of sites affected by radionuclides. These radioactive particles are by-products generated as a result of activities related to nuclear energy and constitute a pollution and a radiotoxicity problem due to its unstable nature of ionizing radiation emissions.
The Chernobyl disaster remains the major and most detrimental nuclear catastrophe which completely altered the radioactive background of the Northern Hemisphere. It happened in April 1986 on the territory of the former Soviet Union. The catastrophe led to the increase of radiation in nearly one million times in some parts of Europe and North America compared to the pre-disaster state Air, water, soils, vegetation and animals were contaminated to a varying degree. Apart from Ukraine and Belarus as the worst hit areas, adversely affected countries included Russia, Austria, Finland and Sweden. The full impact on the aquatic systems, including primarily adjacent valleys of Pripyat river and Dnieper river, are still unexplored.
References
- 1 2 3 Nesterenko, Vassily B.; Yablokov, Alexey V. (2009). "Chapter I. Chernobyl Contamination: An Overview". Annals of the New York Academy of Sciences. 1181 (1): 4–30. Bibcode:2009NYASA1181....4N. doi:10.1111/j.1749-6632.2009.04820.x. ISSN 1749-6632. S2CID 86142366.
- ↑ "Chernobyl | Chernobyl Accident | Chernobyl Disaster - World Nuclear Association". www.world-nuclear.org. Retrieved 2019-04-18.
- 1 2 3 4 5 6 "Chapter II The release, dispersion and deposition of radionuclides - Chernobyl: Assessment of Radiological and Health Impact". www.oecd-nea.org. Retrieved 2019-04-18.
- ↑ "11.5: Vapor Pressure". Chemistry LibreTexts. 2014-11-18. Retrieved 2019-04-18.
- 1 2 "Strontium (Sr) - Chemical properties, Health and Environmental effects". www.lenntech.com. Retrieved 2019-04-18.
- 1 2 ChemiCool Periodic Table of Elements and Chemistry. 2011-03-01.
- ↑ Murphy, D.M.; Froyd, K.D.; Apel, E.; Blake, D.; Blake, N.; Evangeliou, N.; Hornbrook, R.S.; Peischl, J.; Ray, E.; Ryerson, T.B.; Thompson, C.; Stohl, A. (April 2018). "An aerosol particle containing enriched uranium encountered in the remote upper troposphere". Journal of Environmental Radioactivity. 184–185: 95–100. doi:10.1016/j.jenvrad.2018.01.006. PMID 29407642.
- 1 2 "Radon". National Institute of Environmental Health Sciences. Retrieved 2019-04-18.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Smith, Jim; Beresford, Nicholas A. (2005). Chernobyl — Catastrophe and Consequences | SpringerLink. Springer Praxis Books. doi:10.1007/3-540-28079-0. ISBN 978-3-540-23866-9.
- 1 2 3 4 5 6 7 Nesterenko, Vassily B.; Yablokov, Alexey V. (2009). "Chapter I. Chernobyl Contamination: An Overview". Annals of the New York Academy of Sciences. 1181 (1): 4–30. Bibcode:2009NYASA1181....4N. doi:10.1111/j.1749-6632.2009.04820.x. ISSN 1749-6632. S2CID 86142366.
- 1 2 3
- •Moller, A. P.; Mousseau, T. A. (2011). "Conservation consequences of Chernobyl and other nuclear accidents". Biological Conservation . Elsevier Ltd. 144 (12): 2787–2798. doi:10.1016/j.biocon.2011.08.009. ISSN 0006-3207. S2CID 4110805.
- •Child, Michael; Koskinen, Otto; Linnanen, Lassi; Breyer, Christian (2018). "Sustainability guardrails for energy scenarios of the global energy transition". Renewable and Sustainable Energy Reviews . Elsevier Ltd. 91: 321–334. doi:10.1016/j.rser.2018.03.079. ISSN 1364-0321. S2CID 117537591.