Related Research Articles

Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, and metabolism. Over the last decades of the 20th century, biochemistry has become successful at explaining living processes through these three disciplines. Almost all areas of the life sciences are being uncovered and developed through biochemical methodology and research. Biochemistry focuses on understanding the chemical basis which allows biological molecules to give rise to the processes that occur within living cells and between cells, in turn relating greatly to the understanding of tissues and organs as well as organism structure and function. Biochemistry is closely related to molecular biology, the study of the molecular mechanisms of biological phenomena.
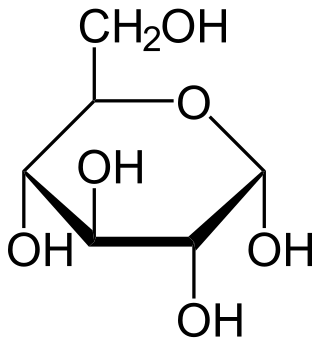
Glucose is a sugar with the molecular formula C6H12O6. Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world.
Insulin is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the insulin (INS) gene. It is considered to be the main anabolic hormone of the body. It regulates the metabolism of carbohydrates, fats and protein by promoting the absorption of glucose from the blood into liver, fat and skeletal muscle cells. In these tissues the absorbed glucose is converted into either glycogen via glycogenesis or fats (triglycerides) via lipogenesis, or, in the case of the liver, into both. Glucose production and secretion by the liver is strongly inhibited by high concentrations of insulin in the blood. Circulating insulin also affects the synthesis of proteins in a wide variety of tissues. It is therefore an anabolic hormone, promoting the conversion of small molecules in the blood into large molecules inside the cells. Low insulin levels in the blood have the opposite effect by promoting widespread catabolism, especially of reserve body fat.

Nitrate is a polyatomic ion with the chemical formula NO−
3. Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble nitrate is bismuth oxynitrate.

Clinical chemistry is a division in medical laboratory sciences focusing on qualitative tests of important compounds, referred to as analytes or markers, in bodily fluids and tissues using analytical techniques and specialized instruments. This interdisciplinary field includes knowledge from medicine, biology, chemistry, biomedical engineering, informatics, and an applied form of biochemistry.

The blood sugar level, blood sugar concentration, blood glucose level, or glycemia is the measure of glucose concentrated in the blood. The body tightly regulates blood glucose levels as a part of metabolic homeostasis.

Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and MnO−
4, an intensely pink to purple solution.

Potassium bitartrate, also known as potassium hydrogen tartrate, with formula KC4H5O6, is a chemical compound with a number of uses.

Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge. The Ser residue is generally in the sequence -Ser-Gly-X-Gly-, although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue.

Potassium perchlorate is the inorganic salt with the chemical formula KClO4. Like other perchlorates, this salt is a strong oxidizer although it usually reacts very slowly with organic substances. This, usually obtained as a colorless, crystalline solid, is a common oxidizer used in fireworks, ammunition percussion caps, explosive primers, and is used variously in propellants, flash compositions, stars, and sparklers. It has been used as a solid rocket propellant, although in that application it has mostly been replaced by the higher performance ammonium perchlorate.

Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic, not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.

Bismuth(III) iodide is the inorganic compound with the formula BiI3. This gray-black salt is the product of the reaction of bismuth and iodine, which once was of interest in qualitative inorganic analysis.

Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs naturally, and its sulfide and oxide forms are important commercial ores. The free element is 86% as dense as lead. It is a brittle metal with a silvery-white color when freshly produced. Surface oxidation generally gives samples of the metal a somewhat rosy cast. Further oxidation under heat can give bismuth a vividly iridescent appearance due to thin-film interference. Bismuth is both the most diamagnetic element and one of the least thermally conductive metals known.

The Sakaguchi test is a chemical test used to detect presence of arginine in proteins. It is named after the Japanese food scientist and organic chemist, Shoyo Sakaguchi (1900–1995) who described the test in 1925. The Sakaguchi reagent used in the test consists of 1-Naphthol and a drop of sodium hypobromite. The guanidino (–C group in arginine reacts with the Sakaguchi reagent to form a red-coloured complex.
The rapid furfural test is a chemical test used to distinguish between glucose and fructose. The rapid furfural test is similar to Molisch's test but uses concentrated hydrochloric acid instead of concentrated sulfuric acid and the solution is boiled. Dilute sugar solution is added to ethanolic 1-naphthol and concentrated hydrochloric acid. The solution is then boiled and if a purple colour forms within thirty seconds, fructose is present. If a purple colour does not appear before thirty seconds, glucose is present.
Heller's test is a chemical test that shows that strong acids cause the denaturation of precipitated proteins. Concentrated nitric acid is added to a protein solution from the side of the test tube to form two layers. A white ring appears between the two layers if the test is positive. Heller's test is commonly used to test for the presence of proteins in urine. This test was discovered by the Austrian Chemist, Johann Florian Heller (1813-1871).
Gunzberg's test is a chemical test used for detecting the presence of hydrochloric acid. Gunzberg's reagent is made by dissolving two grams of phloroglucinol and one gram of vanillin in 100 millilitres of 95% ethanol. Hydrochloric acid catalyses Gunzberg's reagent to form a red complex.
Kelling's test is a chemical test used for detecting the presence of lactic acid in gastric juice.
Gmelin's test is a chemical test used for detecting the presence of bile pigments in urine. It is named after Leopold Gmelin, who introduced the test. Five millilitres of urine is slowly added to five millilitres of concentrated nitric acid in a test-tube. Different coloured rings between the two layers are visible if bile pigments are present as they are oxidised to various chemical products. Nitric acid is used as the oxidising agent. Blue, green and violet rings are seen if bilirubin is present. Gmelin's test is not sensitive, so a positive result always indicates the presence of bile pigments, but a negative result does not exclude the presence of small quantities of bile pigments.
Hay's test, also known as Hay's sulphur powder test, is a chemical test used for detecting the presence of bile salts in urine.
References
- ↑ Dandekar (1 January 2004). Practicals And Viva In Medical Biochemistry. Elsevier India. p. 17. ISBN 978-81-8147-025-6.
- ↑ S. C. Nigam; S C Nigam Omkar (1 January 2006). Experimental Animal Physiology And Biochemistry. New Age International. p. 14. ISBN 978-81-224-1464-6.
- ↑ Srinivas B Rao. Practical Biochemistry for Medical Students. Academic Publishers. p. 19.