
Silver nitrate is an inorganic compound with chemical formula AgNO
3. It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called lunar caustic because silver was called luna by ancient alchemists who associated silver with the moon. In solid silver nitrate, the silver ions are three-coordinated in a trigonal planar arrangement.

Lead(II) nitrate is an inorganic compound with the chemical formula Pb(NO3)2. It commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
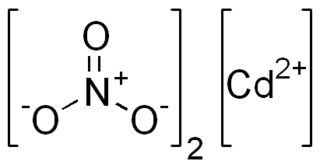
Cadmium nitrate describes any of the related members of a family of inorganic compounds with the general formula Cd(NO3)2·xH2O. The most commonly encountered form being the tetrahydrate.The anhydrous form is volatile, but the others are colourless crystalline solids that are deliquescent, tending to absorb enough moisture from the air to form an aqueous solution. Like other cadmium compounds, cadmium nitrate is known to be carcinogenic. According to X-ray crystallography, the tetrahydrate features octahedral Cd2+ centers bound to six oxygen ligands.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.

Nickel nitrate is the inorganic compound Ni(NO3)2 or any hydrate thereof. In the hexahydrate, the nitrate anions are not bonded to nickel. Other hydrates have also been reported: Ni(NO3)2.9H2O, Ni(NO3)2.4H2O, and Ni(NO3)2.2H2O.

Bismuth chloride (or butter of bismuth) is an inorganic compound with the chemical formula BiCl3. It is a covalent compound and is the common source of the Bi3+ ion. In the gas phase and in the crystal, the species adopts a pyramidal structure, in accord with VSEPR theory.

Bismuth oxynitrate is the name applied to a number of compounds that contain Bi3+, nitrate ions and oxide ions and which can be considered as compounds formed from Bi2O3, N2O5 and H2O. Other names for bismuth oxynitrate include bismuth subnitrate and bismuthyl nitrate. In older texts bismuth oxynitrate is often simply described as BiONO3 or basic bismuth nitrate. Bismuth oxynitrate was once called magisterium bismuti or bismutum subnitricum, and was used as a white pigment, in beauty care, and as a gentle disinfectant for internal and external use. It is also used to form Dragendorff's reagent, which is used as a TLC stain.

Actinide chemistry is one of the main branches of nuclear chemistry that investigates the processes and molecular systems of the actinides. The actinides derive their name from the group 3 element actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide. All but one of the actinides are f-block elements, corresponding to the filling of the 5f electron shell; lawrencium, a d-block element, is also generally considered an actinide. In comparison with the lanthanides, also mostly f-block elements, the actinides show much more variable valence. The actinide series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.

Zirconium nitrate is a volatile anhydrous transition metal nitrate salt of zirconium with formula Zr(NO3)4. It has alternate names of zirconium tetranitrate, or zirconium(IV) nitrate.

Thorium(IV) nitrate is a chemical compound, a salt of thorium and nitric acid with the formula Th(NO3)4. A white solid in its anhydrous form, it can form tetra- and pentahydrates. As a salt of thorium it is weakly radioactive.
Indium(III) nitrate is a nitrate salt of indium which forms various hydrates. Only the pentahydrate has been crystallographically verified. Other hydrates are also reported in literature, such as the trihydrate.

Nitratoauric acid, hydrogen tetranitratoaurate, or simply called gold(III) nitrate is a crystalline gold compound that forms the trihydrate, HAu(NO3)4·3H2O or more correctly H5O2Au(NO3)4·H2O. This compound is an intermediate in the process of extracting gold. In older literature it is also known as aurinitric acid.

Iron(II) nitrate is the nitrate salt of iron(II). It is commonly encountered as the green hexahydrate, Fe(NO3)2·6H2O, which is a metal aquo complex, however it is not commercially available unlike iron(III) nitrate due to its instability to air. The salt is soluble in water serves as a ready source of ferrous ions.

Ytterbium(III) nitrate is an inorganic compound, a salt of ytterbium and nitric acid with the chemical formula Yb(NO3)3. The compound forms colorless crystals, dissolves in water, and also forms crystalline hydrates.
Curium(III) nitrate is an inorganic compound, a salt of curium and nitric acid with the chemical formula Cm(NO3)3.

Thulium(III) nitrate is an inorganic compound, a salt of thulium and nitric acid with the chemical formula Tm(NO3)3. The compound forms dark-green crystals, readily soluble in water, also forms crystalline hydrates.
Ytterbium compounds are chemical compounds that contain the element ytterbium (Yb). The chemical behavior of ytterbium is similar to that of the rest of the lanthanides. Most ytterbium compounds are found in the +3 oxidation state, and its salts in this oxidation state are nearly colorless. Like europium, samarium, and thulium, the trihalides of ytterbium can be reduced to the dihalides by hydrogen, zinc dust, or by the addition of metallic ytterbium. The +2 oxidation state occurs only in solid compounds and reacts in some ways similarly to the alkaline earth metal compounds; for example, ytterbium(II) oxide (YbO) shows the same structure as calcium oxide (CaO).

Bismuth oxyiodide is an inorganic compound, an oxyiodide of bismuth, with the chemical formula BiOI.

Bismuthyl — inorganic oxygen-containing singly charged ion with the chemical formula BiO+, is an oxycation of bismuth in the +3 oxidation state. Most often it is formed during the hydrolysis of trivalent bismuth salts, primarily nitrate, chloride and other halides. In chemical compounds, bismuthyl plays the role of a monovalent cation.




















