
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal. Chromium is the main additive in stainless steel, to which it adds anti-corrosive properties. Chromium is also highly valued as a metal that is able to be highly polished while resisting tarnishing. Polished chromium reflects almost 70% of the visible spectrum, and almost 90% of infrared light. The name of the element is derived from the Greek word χρῶμα, chrōma, meaning color, because many chromium compounds are intensely colored.

Solubility is the property of a solid, liquid or gaseous chemical substance called solute to dissolve in a solid, liquid or gaseous solvent. The solubility of a substance fundamentally depends on the physical and chemical properties of the solute and solvent as well as on temperature, pressure and presence of other chemicals of the solution. The extent of the solubility of a substance in a specific solvent is measured as the saturation concentration, where adding more solute does not increase the concentration of the solution and begins to precipitate the excess amount of solute.

Potassium dichromate, K
2Cr
2O
7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color. The salt is popular in the laboratory because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.

In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

Chromium(III) chloride (also called chromic chloride) describes any of several compounds of with the formula CrCl3 • xH2O, where x can be 0, 5, and 6. The anhydrous compound with the formula CrCl3 is a violet solid. The most common form of the trichloride is the dark green hexahydrate, CrCl3 • 6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions, bright orange when wet and which dissolves in water concomitant with hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser and a carcinogen.

Chromium(II) acetate hydrate, also known as chromous acetate, is the coordination compound with the formula Cr2(CH3CO2)4(H2O)2. This formula is commonly abbreviated Cr2(OAc)4(H2O)2. This red-coloured compound features a quadruple bond. The preparation of chromous acetate once was a standard test of the synthetic skills of students due to its sensitivity to air and the dramatic colour changes that accompany its oxidation. It exists as the dihydrate and the anhydrous forms.

Chromium(III) fluoride is the name for the inorganic compounds with the chemical formula CrF3 as well as several related hydrates. The compound CrF3 is a green crystalline solid that is insoluble in common solvents, but the coloured hydrates [Cr(H2O)6]F3 and [Cr(H2O)6]F3•3H2O are soluble in water. The trihydrate is green, and the hexahydrate is violet. The anhydrous form sublimes at 1100–1200 °C.

Potassium chromate is the inorganic compound with the formula (K2CrO4). This yellow solid is the potassium salt of the chromate anion. It is a common laboratory chemical, whereas sodium chromate is important industrially.

Chromium(II) chloride describes inorganic compounds with the formula CrCl2(H2O)n. The anhydrous solid is white when pure, however commercial samples are often grey or green; it is hygroscopic and readily dissolves in water to give bright blue air-sensitive solutions of the tetrahydrate Cr(H2O)4Cl2. Chromium(II) chloride has no commercial uses but is used on a laboratory-scale for the synthesis of other chromium complexes.
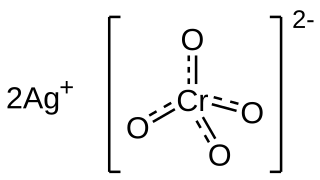
Silver chromate is an inorganic compound with formula Ag2CrO4 which appears as distinctively coloured brown-red crystals. The compound is insoluble and its precipitation is indicative of the reaction between soluble chromate and silver precursor salts (commonly potassium/sodium chromate with silver nitrate). This reaction is important for two uses in the laboratory: in analytical chemistry it constitutes the basis for the Mohr method of argentometry, whereas in neuroscience it is used in the Golgi method of staining neurons for microscopy.

Chromium(III) sulfate usually refers to the inorganic compounds with the formula Cr2(SO4)3.x(H2O), where x can range from 0 to 18. Additionally, ill-defined but commercially important "basic chromium sulfates" are known. These salts are usually either violet or green solids that are soluble in water. It is commonly used in tanning leather.

Chromium(II) sulfate refers to inorganic compounds with the chemical formula CrSO4·n H2O. Several closely related hydrated salts are known. The pentahydrate is a blue solid that dissolves readily in water. Solutions of chromium(II) are easily oxidized by air to Cr(III) species. Solutions of Cr(II) are used as specialized reducing agents of value in organic synthesis.
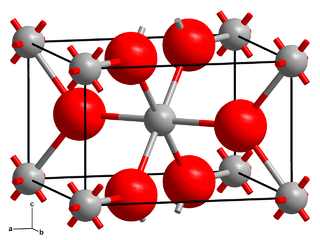
Chromium(II) fluoride is an inorganic compound with the formula CrF2. It exists as a blue-green iridescent solid. Chromium(II) fluoride is sparingly soluble in water, almost insoluble in alcohol, and is soluble in boiling hydrochloric acid, but is not attacked by hot distilled sulfuric acid or nitric acid. Like other chromous compounds, chromium(II) fluoride is oxidized to chromium(III) oxide in air.
Cobalt(III) nitrate is an inorganic compound with the chemical formula Co(NO3)3. A green solid, it exhibits properties of a molecular complex, being sublimable and soluble in chloroform. that sublimates at ambient temperature.

Bismuth(III) nitrate is a salt composed of bismuth in its cationic +3 oxidation state and nitrate anions. The most common solid form is the pentahydrate. It is used in the synthesis of other bismuth compounds. It is available commercially. It is the only nitrate salt formed by a group 15 element, indicative of bismuth's metallic nature.

Chromium(III) phosphate describes inorganic compounds with the chemical formula CrPO4.(H2O)n, where n = 0, 4, or 6. All are deeply colored solids. Anhydrous CrPO4 is green. The hexahydrate CrPO4•6H2O is violet.

Cerium nitrate refers to a family of nitrates of cerium in the three or four oxidation state. Often these compounds contain water, hydroxide, or hydronium ions in addition to cerium and nitrate. Double nitrates of cerium also exist.

Thorium(IV) nitrate is a chemical compound with the formula Th(NO3)4. A white solid in its anhydrous form, it can form tetra- and pentahydrates. As a salt of thorium it is weakly radioactive.

Chromium(III) acetate, commonly known as basic chromium acetate, describes a family of salts where the cation has the formula [Cr3O(O2CCH3)6(OH2)3]+. The trichromium cation is encountered with a variety of anions, such as chloride and nitrate. Data in the table above are for the chloride hexahydrate, [Cr3O(O2CCH3)6(OH2)3]Cl(H2O)6.





















