
The nitronium ion, [NO2]+, is a cation. It is an onium ion because its nitrogen atom has +1 charge, similar to ammonium ion [NH4]+. It is created by the removal of an electron from the paramagnetic nitrogen dioxide molecule NO2, or the protonation of nitric acid HNO3.

Nitrogen dioxide is a chemical compound with the formula NO2. One of several nitrogen oxides, nitrogen dioxide is a reddish-brown gas. It is a paramagnetic, bent molecule with C2v point group symmetry. Industrially, NO2 is an intermediate in the synthesis of nitric acid, millions of tons of which are produced each year, primarily for the production of fertilizers.

Dinitrogen pentoxide is the chemical compound with the formula N2O5. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that sublime slightly above room temperature, yielding a colorless gas.
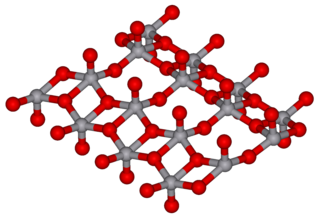
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst.
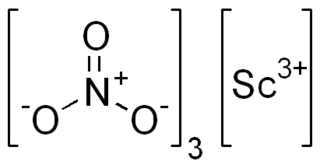
Scandium(III) nitrate, Sc(NO3)3, is an ionic compound. It is an oxidizer, as all nitrates are. The salt is applied in optical coatings, catalysts, electronic ceramics and the laser industry.

Vanadium oxytrichloride is the inorganic compound with the formula VOCl3. This yellow distillable liquid hydrolyzes readily in air. It is an oxidizing agent. It is used as a reagent in organic synthesis. Samples often appear red or orange owing to an impurity of vanadium tetrachloride.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Nickel nitrate is the inorganic compound Ni(NO3)2 or any hydrate thereof. In the hexahydrate, the nitrate anions are not bonded to nickel. Other hydrates have also been reported: Ni(NO3)2.9H2O, Ni(NO3)2.4H2O, and Ni(NO3)2.2H2O.

The vanadyl or oxovanadium(IV) cation, VO2+, is a functional group that is common in the coordination chemistry of vanadium. Complexes containing this functional group are characteristically blue and paramagnetic. A triple bond is proposed to exist between the V4+ and O2− centers. The description of the bonding in the vanadyl ion was central to the development of modern ligand-field theory.

Nitrogen trioxide or nitrate radical is an oxide of nitrogen with formula NO
3, consisting of three oxygen atoms covalently bound to a nitrogen atom. This highly unstable blue compound has not been isolated in pure form, but can be generated and observed as a short-lived component of gas, liquid, or solid systems.
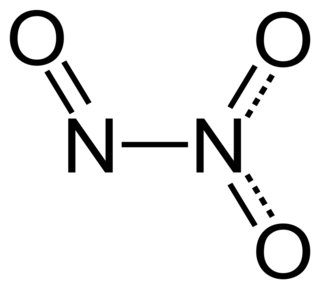
Dinitrogen trioxide is the inorganic compound with the formula N2O3. It is a nitrogen oxide. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C (−6 °F):

Vanadyl acetylacetonate is the chemical compound with the formula VO(acac)2, where acac– is the conjugate base of acetylacetone. It is a blue-green solid that dissolves in polar organic solvents. The coordination complex consists of the vanadyl group, VO2+, bound to two acac– ligands via the two oxygen atoms on each. Like other charge-neutral acetylacetonate complexes, it is not soluble in water.

Cobalt(III) nitrate is an inorganic compound with the chemical formula Co(NO3)3. It is a green, diamagnetic solid that sublimes at ambient temperature.

Titanium nitrate is the inorganic compound with formula Ti(NO3)4. It is a colorless, diamagnetic solid that sublimes readily. It is an unusual example of a volatile binary transition metal nitrate. Ill defined species called titanium nitrate are produced upon dissolution of titanium or its oxides in nitric acid.
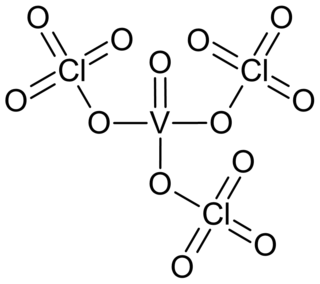
Vanadyl perchlorate or vanadyl triperchlorate is a golden yellow coloured liquid or crystalline compound of vanadium, oxygen and perchlorate group. The substance consists of molecules covalently bound and is quite volatile; it ignites organic solvents on contact and explodes at temperatures above 80 °C.
Vanadium phosphates are inorganic compounds with the formula VOxPO4 as well related hydrates with the formula VOxPO4(H2O)n. Some of these compounds are used commercially as catalysts for oxidation reactions.
Barbara J. Finlayson-Pitts is a Canadian-American atmospheric chemist. She is a professor in the chemistry department at the University of California, Irvine and is the Director of AirUCI Institute. Finlayson-Pitts and James N. Pitts, Jr. are the authors of Chemistry of the Upper and Lower Atmosphere: Theory, Experiments, and Applications (1999). She has been a member of the National Academy of Sciences since 2006 and is the laureate for the 2017 Garvan–Olin Medal. In 2016 she co-chaired the National Academy of Science report "The Future of Atmospheric Chemistry Research"
Indium(III) nitrate is a nitrate salt of indium which forms various hydrates. Only the pentahydrate has been crystallographically verified. Other hydrates are also reported in literature, such as the trihydrate.

Nitratoauric acid, hydrogen tetranitratoaurate, or simply called gold(III) nitrate is a crystalline gold compound that forms the trihydrate, HAu(NO3)4·3H2O or more correctly H5O2Au(NO3)4·H2O. This compound is an intermediate in the process of extracting gold. In older literature it is also known as aurinitric acid.
Rhenium trioxynitrate, also known as rhenium(VII) trioxide nitrate, is a chemical compound with the formula ReO3NO3. It is a white solid that readily hydrolyzes in moist air.














