
Americium is a synthetic chemical element; it has symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element europium and was thus named after the Americas by analogy.
The actinide or actinoid series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Berkelium is a synthetic chemical element; it has symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium.

Curium is a synthetic chemical element; it has symbol Cm and atomic number 96. This transuranic actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium.

Curium(III) oxide is a compound composed of curium and oxygen with the chemical formula Cm2O3. It is a crystalline solid with a unit cell that contains two curium atoms and three oxygen atoms. The simplest synthesis equation involves the reaction of curium(III) metal with O2−: 2 Cm3+ + 3 O2− ---> Cm2O3. Curium trioxide can exist as five polymorphic forms. Two of the forms exist at extremely high temperatures, making it difficult for experimental studies to be done on the formation of their structures. The three other possible forms which curium sesquioxide can take are the body-centered cubic form, the monoclinic form, and the hexagonal form. Curium(III) oxide is either white or light tan in color and, while insoluble in water, is soluble in inorganic and mineral acids. Its synthesis was first recognized in 1955.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.
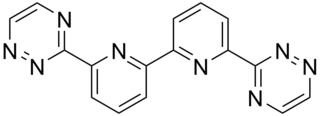
The bis-triazinyl bipyridines (BTBPs) are a class of chemical compounds which are tetradentate ligands similar in shape to quaterpyridine. The BTBPs are made by the reaction of hydrazine and a 1,2-diketone with 6,6'-dicyano-2,2'-bipyridine. The dicyanobipy can be made by reacting 2,2'-bipy with hydrogen peroxide in acetic acid, to form 2,2'-bipyridine-N,N-dioxide. The 2,2'-bipyridine-N,N-dioxide is then converted into the dicyano compound by treatment with potassium cyanide and benzoyl chloride in a mixture of water and THF.

Cerium nitrate refers to a family of nitrates of cerium in the +3 or +4 oxidation state. Often these compounds contain water, hydroxide, or hydronium ions in addition to cerium and nitrate. Double nitrates of cerium also exist.

Titanium nitrate is the inorganic compound with formula Ti(NO3)4. It is a colorless, diamagnetic solid that sublimes readily. It is an unusual example of a volatile binary transition metal nitrate. Ill defined species called titanium nitrate are produced upon dissolution of titanium or its oxides in nitric acid.

Thorium(IV) nitrate is a chemical compound, a salt of thorium and nitric acid with the formula Th(NO3)4. A white solid in its anhydrous form, it can form tetra- and pentahydrates. As a salt of thorium it is weakly radioactive.
Neptunium(IV) nitrate is an inorganic compound, a salt of neptunium and nitric acid with the chemical formula Np(NO3)4. The compound forms gray crystals, dissolves in water, and forms crystal hydrates.
Dysprosium(III) nitrate is an inorganic compound, a salt of dysprosium and nitric acid with the chemical formula Dy(NO3)3. The compound forms yellowish crystals, dissolves in water, forms a crystalline hydrate.

Ytterbium(III) nitrate is an inorganic compound, a salt of ytterbium and nitric acid with the chemical formula Yb(NO3)3. The compound forms colorless crystals, dissolves in water, and also forms crystalline hydrates.
Lutetium(III) nitrate is an inorganic compound, a salt of lutetium and nitric acid with the chemical formula Lu(NO3)3. The compound forms colorless crystals, dissolves in water, and also forms crystalline hydrates. The compound is poisonous.

Thulium(III) nitrate is an inorganic compound, a salt of thulium and nitric acid with the chemical formula Tm(NO3)3. The compound forms dark-green crystals, readily soluble in water, also forms crystalline hydrates.
Curium compounds are compounds containing the element curium (Cm). Curium usually forms compounds in the +3 oxidation state, although compounds with curium in the +4, +5 and +6 oxidation states are also known.

A transition metal nitrate complex is a coordination compound containing one or more nitrate ligands. Such complexes are common starting reagents for the preparation of other compounds.

Berkelium(III) nitrate is the berkelium salt of nitric acid with the formula Bk(NO3)3. It commonly forms the tetrahydrate, Bk(NO3)3·4H2O, which is a light green solid. If heated to 450 °C, it decomposes to berkelium(IV) oxide and 22 milligrams of the solution of this compound is reported to cost one million dollars.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.
Cerium compounds are compounds containing the element cerium (Ce), a lanthanide. Cerium exists in two main oxidation states, Ce(III) and Ce(IV). This pair of adjacent oxidation states dominates several aspects of the chemistry of this element. Cerium(IV) aqueous solutions may be prepared by reacting cerium(III) solutions with the strong oxidizing agents peroxodisulfate or bismuthate. The value of E⦵(Ce4+/Ce3+) varies widely depending on conditions due to the relative ease of complexation and hydrolysis with various anions, although +1.72 V is representative. Cerium is the only lanthanide which has important aqueous and coordination chemistry in the +4 oxidation state.















