In organic chemistry, a carbanion is an anion in which carbon is negatively charged.
In chemistry, a hypervalent molecule is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus pentachloride, sulfur hexafluoride, chlorine trifluoride, the chlorite ion, and the triiodide ion are examples of hypervalent molecules.

Valence shell electron pair repulsion (VSEPR) theory is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm.
The 1,3-dipolar cycloaddition is a chemical reaction between a 1,3-dipole and a dipolarophile to form a five-membered ring. The earliest 1,3-dipolar cycloadditions were described in the late 19th century to the early 20th century, following the discovery of 1,3-dipoles. Mechanistic investigation and synthetic application were established in the 1960s, primarily through the work of Rolf Huisgen. Hence, the reaction is sometimes referred to as the Huisgen cycloaddition. 1,3-dipolar cycloaddition is an important route to the regio- and stereoselective synthesis of five-membered heterocycles and their ring-opened acyclic derivatives. The dipolarophile is typically an alkene or alkyne, but can be other pi systems. When the dipolarophile is an alkyne, aromatic rings are generally produced.

Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides/oxychlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride.

Methyllithium is the simplest organolithium reagent, with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in solution with an ether as the solvent, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive toward water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers.

The Bürgi–Dunitz angle is one of two angles that fully define the geometry of "attack" of a nucleophile on a trigonal unsaturated center in a molecule, originally the carbonyl center in an organic ketone, but now extending to aldehyde, ester, and amide carbonyls, and to alkenes (olefins) as well. The angle was named after crystallographers Hans-Beat Bürgi and Jack D. Dunitz, its first senior investigators.

Hexamethyltungsten is the chemical compound W(CH3)6 also written WMe6. Classified as a transition metal alkyl complex, hexamethyltungsten is an air-sensitive, red, crystalline solid at room temperature; however, it is extremely volatile and sublimes at −30 °C. Owing to its six methyl groups it is extremely soluble in petroleum, aromatic hydrocarbons, ethers, carbon disulfide, and carbon tetrachloride.
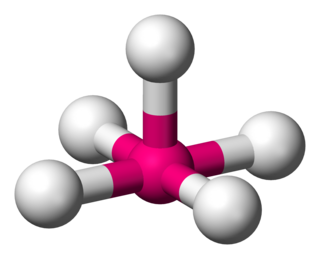
Square pyramidal geometry describes the shape of certain chemical compounds with the formula ML5 where L is a ligand. If the ligand atoms were connected, the resulting shape would be that of a pyramid with a square base. The point group symmetry involved is of type C4v. The geometry is common for certain main group compounds that have a stereochemically-active lone pair, as described by VSEPR theory. Certain compounds crystallize in both the trigonal bipyramidal and the square pyramidal structures, notably [Ni(CN)5]3−.

Organobismuth chemistry is the chemistry of organometallic compounds containing a carbon to bismuth chemical bond. Applications are few. The main bismuth oxidation states are Bi(III) and Bi(V) as in all higher group 15 elements. The energy of a bond to carbon in this group decreases in the order P > As > Sb > Bi. The first reported use of bismuth in organic chemistry was in oxidation of alcohols by Frederick Challenger in 1934 (using Ph3Bi(OH)2). Knowledge about methylated species of bismuth in environmental and biological media is limited.

Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common.

Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929, Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity.
In chemistry, molecular oxohalides (oxyhalides) are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. They have the general formula AOmXn, where X is a halogen. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I) in their molecules. The element A may be a main group element, a transition element, a rare earth element or an actinide. The term oxohalide, or oxyhalide, may also refer to minerals and other crystalline substances with the same overall chemical formula, but having an ionic structure.

Transition metal alkyl complexes are coordination complexes that contain a bond between a transition metal and an alkyl ligand. Such complexes are not only pervasive but are of practical and theoretical interest.
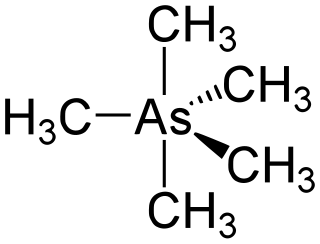
Pentamethylarsenic (or pentamethylarsorane)is an organometalllic compound containing five methyl groups bound to an arsenic atom with formula As(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid.

Pentamethylantimony or pentamethylstiborane is an organometalllic compound containing five methyl groups bound to an antimony atom with formula Sb(CH3)5. It is an example of a hypervalent compound. The molecular shape is trigonal bipyramid. Some other antimony(V) organometallic compounds include pentapropynylantimony (Sb(CCCH3)5) and pentaphenyl antimony (Sb(C6H5)5). Other known pentamethyl-pnictides include pentamethylbismuth and pentamethylarsenic.

Pentamethyltantalum is a homoleptic organotantalum compound. It has a propensity to explode when it is melted. Its discovery was part of a sequence that lead to Richard R. Schrock's Nobel Prize discovery in olefin metathesis.

Tellurophenes are the tellurium analogue of thiophenes and selenophenes.

(Pentamethylcyclopentadienyl)aluminium(I) is an organometallic compound with the formula Al(C5Me5) ("Me" is a methyl group; CH3). The compound is often abbreviated to AlCp* or Cp*Al, where Cp* is the pentamethylcyclopentadienide anion (C5Me5−). Discovered in 1991 by Dohmeier et al., AlCp* serves as the first ever documented example of a room temperature stable monovalent aluminium compound. In its isolated form, Cp*Al exists as the tetramer [Cp*Al]4, and is a yellow crystal that decomposes at temperatures above 100 °C but also sublimes at temperatures above 140 °C.

Nontrigonal pnictogen compounds refer to tricoordinate trivalent pnictogen compounds that are not of typical trigonal pyramidal molecular geometry. By virtue of their geometric constraint, these compounds exhibit distinct electronic structures and reactivities, which bestow on them potential to provide unique nonmetal platforms for bond cleavage reactions.
















