
In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.

In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to an organyl group, or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two organyl groups. They have the general formula R−O−R′, where R and R′ represent organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

Methyl radical is an organic compound with the chemical formula CH•
3. It is a metastable colourless gas, which is mainly produced in situ as a precursor to other hydrocarbons in the petroleum cracking industry. It can act as either a strong oxidant or a strong reductant, and is quite corrosive to metals.
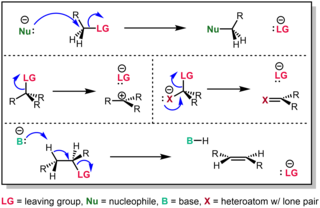
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term nucleofuge. In this context, leaving groups are generally anions or neutral species, departing from neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known.

In organic chemistry, an ethyl group is an alkyl substituent with the formula −CH2CH3, derived from ethane. Ethyl is used in the International Union of Pure and Applied Chemistry's nomenclature of organic chemistry for a saturated two-carbon moiety in a molecule, while the prefix "eth-" is used to indicate the presence of two carbon atoms in the molecule.
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons.
In organic chemistry, a carbanion is an anion in which carbon is negatively charged.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.

In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure R−CH2−C6H5. Benzyl features a benzene ring attached to a methylene group group.
![<span class="mw-page-title-main">Chemical ionization</span> Ionization technique used in mass [[spectroscopy]]](https://upload.wikimedia.org/wikipedia/commons/thumb/7/7b/Chemical_Ionization.png/320px-Chemical_Ionization.png)
Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules are ionized by electron ionization to form reagent ions, which subsequently react with analyte molecules in the gas phase to create analyte ions for analysis by mass spectrometry. Negative chemical ionization (NCI), charge-exchange chemical ionization, atmospheric-pressure chemical ionization (APCI) and atmospheric pressure photoionization (APPI) are some of the common variants of the technique. CI mass spectrometry finds general application in the identification, structure elucidation and quantitation of organic compounds as well as some utility in biochemical analysis. Samples to be analyzed must be in vapour form, or else, must be vapourized before introduction into the source.
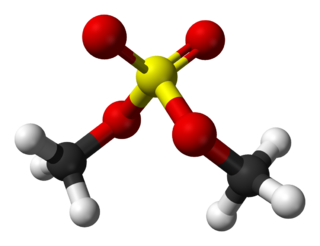
Dimethyl sulfate (DMS) is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as (CH3)2SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agent in organic synthesis.
![<span class="mw-page-title-main">Sulfonium</span> Cation of the form [SR3]+](https://upload.wikimedia.org/wikipedia/commons/thumb/6/62/%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png/320px-%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png)
In organic chemistry, a sulfonium ion, also known as sulphonium ion or sulfanium ion, is a positively-charged ion featuring three organic substituents attached to sulfur. These organosulfur compounds have the formula [SR3]+. Together with a negatively-charged counterion, they give sulfonium salts. They are typically colorless solids that are soluble in organic solvent.

Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group [R−N+≡N]X− where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.

Trimethylsilyldiazomethane is the organosilicon compound with the formula (CH3)3SiCHN2. It is classified as a diazo compound. Trimethylsilyldiazomethane is a commercially available reagent used in organic chemistry as a methylating agent and as a source of CH2 group. Its behavior is akin to the less convenient reagent diazomethane.
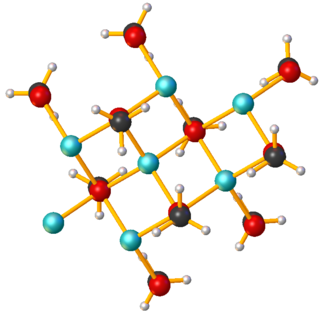
Sodium methoxide is the simplest sodium alkoxide. With the formula CH3ONa, it is a white solid, which is formed by the deprotonation of methanol. It is a widely used reagent in industry and the laboratory. It is also a dangerously caustic base.
In chemistry, a reaction intermediate, or intermediate, is a molecular entity arising within the sequence of a stepwise chemical reaction. It is formed as the reaction product of an elementary step, from the reactants and/or preceding intermediates, but is consumed in a later step. It does not appear in the chemical equation for the overall reaction.
Methylene is an organic compound with the chemical formula CH
2. It is a colourless gas that fluoresces in the mid-infrared range, and only persists in dilution, or as an adduct.
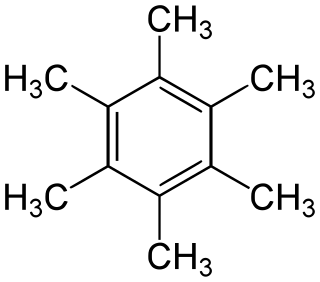
Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929, Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity.
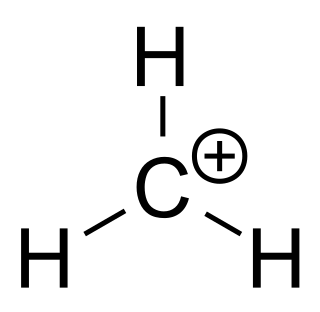
In organic chemistry, methenium is a cation with the formula CH+
3. It can be viewed as a methylene radical with an added proton, or as a methyl radical with one electron removed. It is a carbocation and an enium ion, making it the simplest of the carbenium ions.









![<span class="mw-page-title-main">Chemical ionization</span> Ionization technique used in mass [[spectroscopy]]](https://upload.wikimedia.org/wikipedia/commons/thumb/7/7b/Chemical_Ionization.png/320px-Chemical_Ionization.png)

![<span class="mw-page-title-main">Sulfonium</span> Cation of the form [SR3]+](https://upload.wikimedia.org/wikipedia/commons/thumb/6/62/%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png/320px-%28CH3%293S%2B_in_the_BPh4-_salt_%28code_HEYZAM%29.png)





