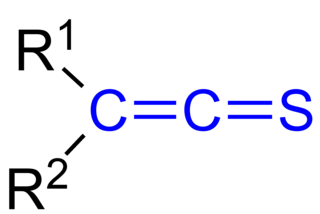In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group bound to a saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sucrose and cholesterol. The presence of an OH group strongly modifies the properties of hydrocarbons, conferring hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.
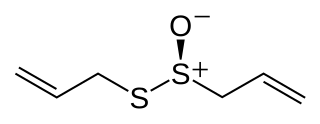
Allicin is an organosulfur compound obtained from garlic. When fresh garlic is chopped or crushed, the enzyme alliinase converts alliin into allicin, which is responsible for the aroma of fresh garlic. Allicin is unstable and quickly changes into a series of other sulfur-containing compounds such as diallyl disulfide. Allicin is an antifeedant, i.e. the defense mechanism against attacks by pests on the garlic plant.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.

In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.
Asparagusic acid is an organosulfur compound with the molecular formula C4H6O2S2 and systematically named 1,2-dithiolane-4-carboxylic acid. The molecule consists of a heterocyclic disulfide functional group (a 1,2-dithiolane) with a carboxylic acid side chain. It is found in asparagus and is believed to be the metabolic precursor to odorous sulfur compounds responsible for the distinctive smell of urine which has long been associated with eating asparagus.

In chemistry, a sulfenic acid is an organosulfur compound and oxoacid with the general formula R−S−OH. It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids and sulfonic acids, respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide.
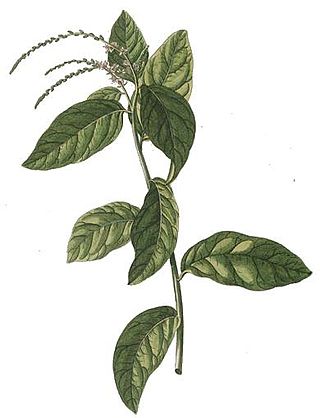
Petiveria is a genus of flowering plants in the pigeonberry family, Petiveriaceae. The sole species it contains, Petiveria alliacea, is native to Florida and the lower Rio Grande Valley of Texas in the United States, Mexico, Central America, the Caribbean, and tropical South America. Introduced populations occur in Benin and Nigeria. It is a deeply rooted herbaceous perennial shrub growing up to 1 m (3.3 ft) in height and has small greenish piccate flowers. The roots and leaves have a strong acrid, garlic-like odor which taints the milk and meat of animals that graze on it.

In organic chemistry, a dithiocarbamate is a functional group with the general formula R2N−C(=S)−S−R and structure >N−C(=S)−S−. It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms.

Hexamethyltungsten is the chemical compound W(CH3)6 also written WMe6. Classified as a transition metal alkyl complex, hexamethyltungsten is an air-sensitive, red, crystalline solid at room temperature; however, it is extremely volatile and sublimes at −30 °C. Owing to its six methyl groups it is extremely soluble in petroleum, aromatic hydrocarbons, ethers, carbon disulfide, and carbon tetrachloride.

syn-Propanethial S-oxide (or (Z)-propanethial S-oxide), a member of a class of organosulfur compounds known as thiocarbonyl S-oxides (formerly "sulfines"), is a volatile liquid that acts as a lachrymatory agent (triggers tearing and stinging on contact with the eyes). The chemical is released from onions, Allium cepa, as they are sliced. The release is due to the breaking open of the onion cells and their releasing enzymes called alliinases, which then break down amino acid sulfoxides, generating sulfenic acids. A specific sulfenic acid, 1-propenesulfenic acid, formed when onions are cut, is rapidly rearranged by a second enzyme, called the lachrymatory factor synthase or LFS, giving syn-propanethial S-oxide. The gas diffuses through the air and, on contact with the eye, it stimulates sensory neurons creating a stinging, painful sensation. Tears are released from the tear glands to dilute and flush out the irritant. A structurally related lachrymatory compound, syn-butanethial S-oxide, C4H8OS, has been found in another genus Allium plant, Allium siculum.
An insertion reaction is a chemical reaction where one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.
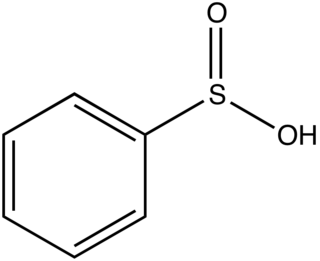
Phenylsulfinic acid is an organosulfur compound with the formula C6H5SO2H. It is a colorless or white crystalline solid that is usually stored in the form of its sodium salt. In aqueous solution it is strongly acidic and is easily oxidized in air. Phenylsulfinic acid and its esters are chiral.
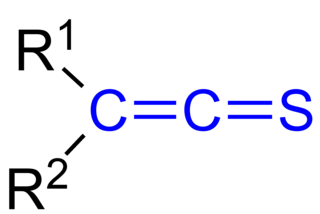
In organic chemistry, thioketenes are organosulfur compounds analogous to ketenes with the general formula R2C=C=S, where R is alkyl or aryl. The parent thioketene (ethenthione) has the formula CH2=C=S. It is the simplest thioketene. Thioketene is stable as a gas, but like most thioketenes, it polymerizes upon condensation.

Sulfinyl halide have the general formula R−S(O)−X, where X is a halogen. They are intermediate in oxidation level between sulfenyl halides, R−S−X, and sulfonyl halides, R−SO2−X. The best known examples are sulfinyl chlorides, thermolabile, moisture-sensitive compounds, which are useful intermediates for preparation of other sufinyl derivatives such as sulfinamides, sulfinates, sulfoxides, and thiosulfinates. Unlike the sulfur atom in sulfonyl halides and sulfenyl halides, the sulfur atom in sulfinyl halides is chiral, as shown for methanesulfinyl chloride.

A seleninic acid is an organoselenium compound and an oxoacid with the general formula RSeO2H, where R ≠ H. Its structure is R−Se(=O)−OH. It is a member of the family of organoselenium oxoacids, which also includes selenenic acids and selenonic acids, which are R−Se−OH and R−Se(=O)2−OH, respectively. The parent member of this family of compounds is methaneseleninic acid, also known as methylseleninic acid or "MSA".

Sulfinylmethane or sulfine is an organic compound with molecular formula H2CSO. It is the simplest sulfine. Sulfines are chemical compounds with the general structure XY=SO. IUPAC considers the term 'sulfine' obsolete, preferring instead thiocarbonyl S-oxide; despite this, the use of the term sulfine still predominates in the chemical literature.