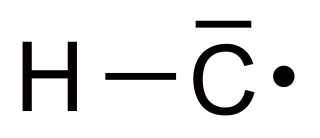Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesized metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG).

Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All genomes sequenced to date encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it (or a thioester) as a substrate. In humans, CoA biosynthesis requires cysteine, pantothenate (vitamin B5), and adenosine triphosphate (ATP).

In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group or hydrogen in the case of formyl group. In organic chemistry, the acyl group is usually derived from a carboxylic acid, in which case it has the formula R−C(=O)−, where R represents an organyl group or hydrogen. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acids and phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond.
Pyruvic acid (IUPAC name: 2-oxopropanoic acid, also called acetoic acid) (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.

Acetyl-CoA is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle to be oxidized for energy production.

An acetate is a salt formed by the combination of acetic acid with a base. "Acetate" also describes the conjugate base or ion typically found in aqueous solution and written with the chemical formula C
2H
3O−
2. The neutral molecules formed by the combination of the acetate ion and a positive ion are also commonly called "acetates". The simplest of these is hydrogen acetate with corresponding salts, esters, and the polyatomic anion CH
3CO−
2, or CH
3COO−
.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.
In chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed acetate esters or simply acetates. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound.

Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air.

Acetyl chloride is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.

An organic acid anhydride is an acid anhydride that is also an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word acid in the name of the parent carboxylic acid by the word anhydride. Thus, (CH3CO)2O is called acetic anhydride.Mixed (or unsymmetrical) acid anhydrides, such as acetic formic anhydride (see below), are known, whereby reaction occurs between two different carboxylic acids. Nomenclature of unsymmetrical acid anhydrides list the names of both of the reacted carboxylic acids before the word "anhydride" (for example, the dehydration reaction between benzoic acid and propanoic acid would yield "benzoic propanoic anhydride").

Malonyl-CoA is a coenzyme A derivative of malonic acid.
Chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC).
In organic chemistry, the acetoxy group, is a functional group with the formula −OCOCH3 and the structure −O−C(=O)−CH3. As the -oxy suffix implies, it differs from the acetyl group by the presence of an additional oxygen atom. The name acetoxy is the short form of acetyl-oxy.
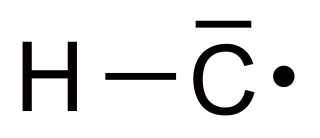
Methylidyne, or (unsubstituted) carbyne, is an organic compound whose molecule consists of a single hydrogen atom bonded to a carbon atom. It is the parent compound of the carbynes, which can be seen as obtained from it by substitution of other functional groups for the hydrogen.

Thioacetic acid is an organosulfur compound with the molecular formula CH3C(O)SH. It is a thioic acid: the sulfur analogue of acetic acid, as implied by the thio- prefix. It is a yellow liquid with a strong thiol-like odor. It is used in organic synthesis for the introduction of thiol groups in molecules.
Acetyl-CoA synthetase (ACS) or Acetate—CoA ligase is an enzyme involved in metabolism of acetate. It is in the ligase class of enzymes, meaning that it catalyzes the formation of a new chemical bond between two large molecules.

In organic chemistry, ethenone is the formal name for ketene, an organic compound with formula C2H2O or H2C=C=O. It is the simplest member of the ketene class. It is an important reagent for acetylations.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. It has been used, as a component of vinegar, throughout history from at least the third century BC.