
A telluroketone is an analog of a ketone in which the oxygen atom has been replaced by a tellurium atom. This change makes the functional group less stable, requiring greater steric and electronic stabilization. [1]

A telluroketone is an analog of a ketone in which the oxygen atom has been replaced by a tellurium atom. This change makes the functional group less stable, requiring greater steric and electronic stabilization. [1]

In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)–. The simplest ketone is acetone, with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

In organic chemistry, an acetal is a functional group with the connectivity R2C(OR')2. Here, the R groups can be organic fragments or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other or not. Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry.

In organic chemistry, a carbonyl group is a functional group with the formula C=O, composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
In carbohydrate chemistry, a pair of anomers is a pair of near-identical stereoisomers that differ at only the anomeric carbon, the carbon that bears the aldehyde or ketone functional group in the sugar's open-chain form. However, in order for anomers to exist, the sugar must be in its cyclic form, since in open-chain form, the anomeric carbon is planar and thus achiral. More formally stated, then, an anomer is an epimer at the hemiacetal/hemiketal carbon in a cyclic saccharide. Anomerization is the process of conversion of one anomer to the other. As is typical for stereoisomeric compounds, different anomers have different physical properties, melting points and specific rotations.
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Dodecahedrane is a chemical compound, a hydrocarbon with formula C20H20, whose carbon atoms are arranged as the vertices (corners) of a regular dodecahedron. Each carbon is bound to three neighbouring carbon atoms and to a hydrogen atom. This compound is one of the three possible Platonic hydrocarbons, the other two being cubane and tetrahedrane.
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction.

Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen, respectively.
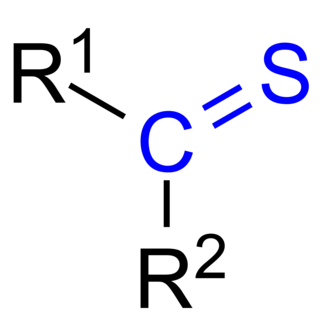
In organic chemistry, thioketones are organosulfur compounds related to conventional ketones in which the oxygen has been replaced by a sulfur. Instead of a structure of R2C=O, thioketones have the structure R2C=S, which is reflected by the prefix "thio-" in the name of the functional group. Unhindered alkylthioketones typically tend to form polymers or rings.

The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid but most of the time either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.

In organic chemistry, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic, or have a bicyclic portion as part of the larger ring system, in either case with the two rings connected through the defining single common atom. The one common atom connecting the participating rings distinguishes spiro compounds from other bicyclics: from isolated ring compounds like biphenyl that have no connecting atoms, from fused ring compounds like decalin having two rings linked by two adjacent atoms, and from bridged ring compounds like norbornane with two rings linked by two non-adjacent atoms.
Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a vicinal diol (glycol) is cleaved and instead the two oxygen atoms become double-bonded to their respective carbon atoms. Depending on the substitution pattern in the diol, these carbonyls can be either ketones or aldehydes.

In chemistry, the descriptor geminal refers to the relationship between two atoms or functional groups that are attached to the same atom. A geminal diol, for example, is a diol attached to the same carbon atom, as in methanediol. Also the shortened prefix gem may be applied to a chemical name to denote this relationship, as in a gem-dibromide for "geminal dibromide".

In organic chemistry, a semicarbazone is a derivative of imines formed by a condensation reaction between a ketone or aldehyde and semicarbazide. They are classified as imine derivatives because they are formed from the reaction of an aldehyde or ketone with the terminal -NH2 group of semicarbazide, which behaves very similarly to primary amines.
In organic chemistry, a homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant unit, generally a methylene group. The reactants undergo a homologation when the number of a repeated structural unit in the molecules is increased. The most common homologation reactions increase the number of methylene units in saturated chain within the molecule. For example, the reaction of aldehydes or ketones with diazomethane or methoxymethylenetriphenylphosphine to give the next homologue in the series.
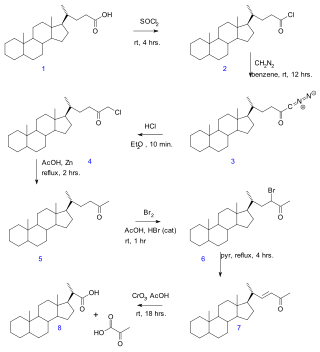
In the Gallagher–Hollander degradation (1946) pyruvic acid is removed from a linear aliphatic carboxylic acid yielding a new acid with two carbon atoms fewer. The original publication concerns the conversion of bile acid in a series of reactions: acid chloride (2) formation with thionyl chloride, diazoketone formation (3) with diazomethane, chloromethyl ketone formation (4) with hydrochloric acid, organic reduction of chlorine to methylketone (5), ketone halogenation to 6, elimination reaction with pyridine to enone 7 and finally oxidation with chromium trioxide to bisnorcholanic acid 8.
Dibenzyl ketone, or 1,3-diphenylacetone, is an organic compound composed of two benzyl groups attached to a central carbonyl group. This results in the central carbonyl carbon atom being electrophilic and the two adjacent carbon atoms slightly nucleophilic. For this reason, dibenzyl ketone is frequently used in an aldol condensation reaction with benzil and base to create tetraphenylcyclopentadienone. Vera Bogdanovskaia is credited with the classification of dibenzyl ketone.

Methoxyketamine or 2-MeO-2-deschloroketamine is a designer drug of the arylcyclohexylamine class first reported in 1963. It is an analog of ketamine in which the chlorine atom has been replaced with a methoxy group. Its synthesis by rearrangement of an amino ketone has been reported. As an arylcyclohexylamine, methoxyketamine most likely functions as an NMDA receptor antagonist. It produces sedative, hallucinogenic, and anesthetic effects, but with a lower potency than ketamine itself.
In organic chemistry, the Davis oxidation or Davis' oxaziridine oxidation refers to oxidations involving the use of the Davis reagent or other similar oxaziridine reagents. This reaction mainly refers to the generation of α-hydroxy carbonyl compounds (acyloins) from ketones or esters. The reaction is carried out in a basic environment to generate the corresponding enolate from the ketone or ester. This reaction has been shown to work for amides.