
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group bound to a saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of hydrocarbons, conferring hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur.
Infrared astronomy is a sub-discipline of astronomy which specializes in the observation and analysis of astronomical objects using infrared (IR) radiation. The wavelength of infrared light ranges from 0.75 to 300 micrometers, and falls in between visible radiation, which ranges from 380 to 750 nanometers, and submillimeter waves.

Astrochemistry is the study of the abundance and reactions of molecules in the universe, and their interaction with radiation. The discipline is an overlap of astronomy and chemistry. The word "astrochemistry" may be applied to both the Solar System and the interstellar medium. The study of the abundance of elements and isotope ratios in Solar System objects, such as meteorites, is also called cosmochemistry, while the study of interstellar atoms and molecules and their interaction with radiation is sometimes called molecular astrophysics. The formation, atomic and chemical composition, evolution and fate of molecular gas clouds is of special interest, because it is from these clouds that solar systems form.

The Pioneer Venus project was part of the Pioneer program consisting of two spacecraft, the Pioneer Venus Orbiter and the Pioneer Venus Multiprobe, launched to Venus in 1978. The program was managed by NASA's Ames Research Center.

The W. M. Keck Observatory is an astronomical observatory with two telescopes at an elevation of 4,145 meters (13,600 ft) near the summit of Mauna Kea in the U.S. state of Hawaii. Both telescopes have 10 m (33 ft) aperture primary mirrors, and, when completed in 1993 and 1996, they were the largest optical reflecting telescopes in the world. They are currently the third and fourth largest.

The Upper Atmosphere Research Satellite (UARS) was a NASA-operated orbital observatory whose mission was to study the Earth's atmosphere, particularly the protective ozone layer. The 5,900-kilogram (13,000 lb) satellite was deployed from Space Shuttle Discovery during the STS-48 mission on 15 September 1991. It entered Earth orbit at an operational altitude of 600 kilometers (370 mi), with an orbital inclination of 57 degrees.

The hydroxyl radical, •HO, is the neutral form of the hydroxide ion (HO–). Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also an important radical formed in radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and SCC in coolant systems subjected to radioactive environments. Hydroxyl radicals are also produced during UV-light dissociation of H2O2 (suggested in 1879) and likely in Fenton chemistry, where trace amounts of reduced transition metals catalyze peroxide-mediated oxidations of organic compounds.

Cabeus is a lunar impact crater that is located about 100 km (62 mi) from the south pole of the Moon. At this location the crater is seen obliquely from Earth, and it is almost perpetually in deep shadow due to lack of sunlight. Hence, not much detail can be seen of this crater, even from orbit. Through a telescope, this crater appears near the southern limb of the Moon, to the west of the crater Malapert and to the south-southwest of Newton.

Airglow is a faint emission of light by a planetary atmosphere. In the case of Earth's atmosphere, this optical phenomenon causes the night sky never to be completely dark, even after the effects of starlight and diffused sunlight from the far side are removed. This phenomenon originates with self-illuminated gases and has no relationship with Earth's magnetism or sunspot activity.
Lunar water is water that is present on the Moon. Diffuse water molecules in low concentrations can persist at the Moon's sunlit surface, as discovered by the SOFIA observatory in 2020. Gradually, water vapor is decomposed by sunlight, leaving hydrogen and oxygen lost to outer space. Scientists have found water ice in the cold, permanently shadowed craters at the Moon's poles. Water molecules are also present in the extremely thin lunar atmosphere.
The study of extraterrestrial atmospheres is an active field of research, both as an aspect of astronomy and to gain insight into Earth's atmosphere. In addition to Earth, many of the other astronomical objects in the Solar System have atmospheres. These include all the gas giants, as well as Mars, Venus and Titan. Several moons and other bodies also have atmospheres, as do comets and the Sun. There is evidence that extrasolar planets can have an atmosphere. Comparisons of these atmospheres to one another and to Earth's atmosphere broaden our basic understanding of atmospheric processes such as the greenhouse effect, aerosol and cloud physics, and atmospheric chemistry and dynamics.

Whether there is life on Titan, the largest moon of Saturn, is currently an open question and a topic of scientific assessment and research. Titan is far colder than Earth, but of all the places in the Solar System, Titan is the only place besides Earth known to have liquids in the form of rivers, lakes, and seas on its surface. Its thick atmosphere is chemically active and rich in carbon compounds. On the surface there are small and large bodies of both liquid methane and ethane, and it is likely that there is a layer of liquid water under its ice shell. Some scientists speculate that these liquid mixes may provide prebiotic chemistry for living cells different from those on Earth.

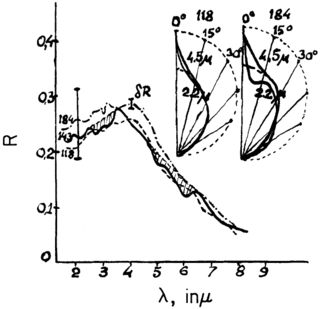
The possibility of life on Venus is a subject of interest in astrobiology due to Venus' proximity and similarities to Earth. To date, no definitive evidence has been found of past or present life there. In the early 1960s, studies conducted via spacecraft demonstrated that the current Venusian environment is extreme compared to Earth's. Studies continue to question whether life could have existed on the planet's surface before a runaway greenhouse effect took hold, and whether a relict biosphere could persist high in the modern Venusian atmosphere.
WASP-33b is an extrasolar planet orbiting the star HD 15082. It was the first planet discovered to orbit a Delta Scuti variable star. With a semimajor axis of 0.026 AU and a mass likely greater than Jupiter's, it belongs to the hot Jupiter class of planets.

Jovian Infrared Auroral Mapper (JIRAM) is an instrument on the Juno spacecraft in orbit of the planet Jupiter. It is an image spectrometer and was contributed by Italy. Similar instruments are on ESA Rosetta, Venus Express, and Cassini-Huygens missions. The primary goal of JIRAM is to probe the upper layers of Jupiter's atmosphere down to pressures of 5–7 bars at infrared wavelengths in the 2–5 μm interval using an imager and a spectrometer. The Jupiter's atmosphere and auroral regions are targeted for study. In particular it has been designed to study the dynamics and chemistry in the atmosphere, perhaps determining the how Jovian hot spots form.
Venus Origins Explorer (VOX) is a concept orbiter mission to Venus.

The Mapping Imaging Spectrometer for Europa (MISE) is an imaging near infrared spectrometer on board the Europa Clipper mission to Jupiter's moon Europa. MISE will examine Europa's surface composition and relate it to the habitability of its internal water ocean.
Nadir and Occultation for MArs Discovery (NOMAD) is a 3-channel spectrometer on board the ExoMars Trace Gas Orbiter (TGO) launched to Mars orbit on 14 March 2016.
CubeSat UV Experiment (CUVE) is a space mission concept to study the atmospheric processes of the planet Venus with a small satellite. Specifically, the orbiter mission would study an enigmatic ultraviolet light absorber of unknown composition situated within the planet's uppermost cloud layer that absorbs about half the solar radiation downwelling in the planet's atmosphere.
Lunar Trailblazer is a planned small lunar orbiter, part of NASA's SIMPLEx program, that will detect and map water on the lunar surface to determine how its form, abundance, and location relate to geology. Its mission is to aid in the understanding of lunar water and the Moon's water cycle. Lunar Trailblazer is currently slated to launch in 2024 as a secondary payload on the IM-2 mission. The Principal Investigator (PI) of the mission is Bethany Ehlmann, a professor at Caltech.















