
A carbohydrate is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 and thus with the empirical formula Cm(H2O)n, which does not mean the H has covalent bonds with O. However, not all carbohydrates conform to this precise stoichiometric definition, nor are all chemicals that do conform to this definition automatically classified as carbohydrates.

In organic chemistry, a ketone is a functional group with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.
Monosaccharides, also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
In chemistry, a pentose is a monosaccharide with five carbon atoms. The chemical formula of many pentoses is C
5H
10O
5, and their molecular weight is 150.13 g/mol.

In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.

In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.

Glyceraldehyde (glyceral) is a triose monosaccharide with chemical formula C3H6O3. It is the simplest of all common aldoses. It is a sweet, colorless, crystalline solid that is an intermediate compound in carbohydrate metabolism. The word comes from combining glycerol and aldehyde, as glyceraldehyde is glycerol with one alcohol group oxidized to an aldehyde.
The aldol reaction is a reaction that combines two carbonyl compounds to form a new β-hydroxy carbonyl compound.

In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and biochemistry. The use of Fischer projections in non-carbohydrates is discouraged, as such drawings are ambiguous and easily confused with other types of drawing. The main purpose of Fischer projections is to show the chirality of a molecule and to distinguish between a pair of enantiomers. Some notable uses include drawing sugars and depicting isomers.
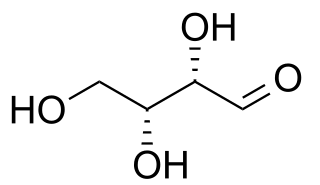
In stereochemistry, diastereomers are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two.
A tetrose is a monosaccharide with 4 carbon atoms. They have either an aldehyde functional group in position 1 (aldotetroses) or a ketone functional group in position 2 (ketotetroses).
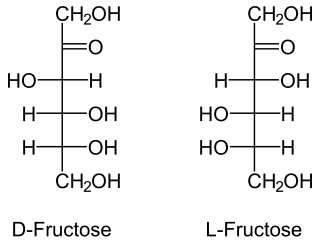
A ketose is a monosaccharide containing one ketone group per molecule. The simplest ketose is dihydroxyacetone, which has only three carbon atoms. It is the only ketose with no optical activity. All monosaccharide ketoses are reducing sugars, because they can tautomerize into aldoses via an enediol intermediate, and the resulting aldehyde group can be oxidised, for example in the Tollens' test or Benedict's test. Ketoses that are bound into glycosides, for example in the case of the fructose moiety of sucrose, are nonreducing sugars.
In carbohydrate chemistry, a pair of anomers is a pair of near-identical stereoisomers or diastereomers that differ at only the anomeric carbon, the carbon that bears the aldehyde or ketone functional group in the sugar's open-chain form. However, in order for anomers to exist, the sugar must be in its cyclic form, since in open-chain form, the anomeric carbon is planar and thus achiral. More formally stated, then, an anomer is an epimer at the hemiacetal/hemiketal carbon in a cyclic saccharide. Anomerization is the process of conversion of one anomer to the other. As is typical for stereoisomeric compounds, different anomers have different physical properties, melting points and specific rotations.
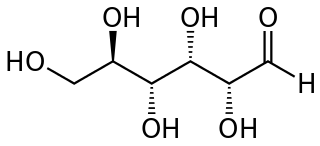
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reaction, the sugar becomes a carboxylic acid.
Aldaric acids are a group of sugar acids, where the terminal hydroxyl and carbonyl groups of the sugars have been replaced by terminal carboxylic acids, and are characterised by the formula HOOC-(CHOH)n-COOH.

Threose is a four-carbon monosaccharide with molecular formula C4H8O4. It has a terminal aldehyde group rather than a ketone in its linear chain, and so is considered part of the aldose family of monosaccharides. The threose name can be used to refer to both the D- and L-stereoisomers, and more generally to the racemic mixture (D/L-, equal parts D- and L-) as well as to the more generic threose structure (absolute stereochemistry unspecified).

Transketolase is an enzyme that, in humans, is encoded by the TKT gene. It participates in both the pentose phosphate pathway in all organisms and the Calvin cycle of photosynthesis. Transketolase catalyzes two important reactions, which operate in opposite directions in these two pathways. In the first reaction of the non-oxidative pentose phosphate pathway, the cofactor thiamine diphosphate accepts a 2-carbon fragment from a 5-carbon ketose (D-xylulose-5-P), then transfers this fragment to a 5-carbon aldose (D-ribose-5-P) to form a 7-carbon ketose (sedoheptulose-7-P). The abstraction of two carbons from D-xylulose-5-P yields the 3-carbon aldose glyceraldehyde-3-P. In the Calvin cycle, transketolase catalyzes the reverse reaction, the conversion of sedoheptulose-7-P and glyceraldehyde-3-P to pentoses, the aldose D-ribose-5-P and the ketose D-xylulose-5-P.
The Kiliani–Fischer synthesis, named for German chemists Heinrich Kiliani and Emil Fischer, is a method for synthesizing monosaccharides. It proceeds via synthesis and hydrolysis of a cyanohydrin, followed by reduction of the intermediate acid to the aldehyde, thus elongating the carbon chain of an aldose by one carbon atom while preserving stereochemistry on all the previously present chiral carbons. The new chiral carbon is produced with both stereochemistries, so the product of a Kiliani–Fischer synthesis is a mixture of two diastereomeric sugars, called epimers. For example, D-arabinose is converted to a mixture of D-glucose and D-mannose.
In carbohydrate chemistry, the Lobry de Bruyn–Van Ekenstein transformation also known as the Lobry de Bruyn–Alberda van Ekenstein transformation is the base or acid catalyzed transformation of an aldose into the ketose isomer or vice versa, with a tautomeric enediol as reaction intermediate. Ketoses may be transformed into 3-ketoses, etcetera. The enediol is also an intermediate for the epimerization of an aldose or ketose.
Monosaccharide nomenclature is the naming system of the building blocks of carbohydrates, the monosaccharides, which may be monomers or part of a larger polymer. Monosaccharides are subunits that cannot be further hydrolysed in to simpler units. Depending on the number of carbon atom they are further classified into trioses, tetroses, pentoses, hexoses etc., which is further classified in to aldoses and ketoses depending on the type of functional group present in them.











