
In organic chemistry, a sulfide or thioether is an organosulfur functional group with the connectivity R−S−R' as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

In organic chemistry, sulfonic acid refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH, where R is an organic alkyl or aryl group and the S(=O)2(OH) group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound is the parent sulfonic acid, HS(=O)2(OH), a tautomer of sulfurous acid, S(=O)(OH)2. Salts or esters of sulfonic acids are called sulfonates.

A peroxy acid is an acid which contains an acidic –OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the peroxy derivatives of organic carboxylic acids. They are generally strong oxidizers.
Organosulfur chemistry is the study of the properties and synthesis of organosulfur compounds, which are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature is abound with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.

In organosulfur chemistry, a sulfonyl group can refer either to a functional group found primarily in sulfones, or to a substituent obtained from a sulfonic acid by the removal of the hydroxyl group, similarly to acyl groups. Sulfonyl groups can be written as having the general formula R−S(=O)2−R′, where there are two double bonds between the sulfur and oxygen.

In organic chemistry, a carbodiimide is a functional group with the formula RN=C=NR. On Earth they are exclusively synthetic, but in interstellar space the parent compound HN=C=NH has been detected by its maser emissions.

In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and dimethyl sulfoxide (DMSO), a common solvent.
Sulfur compounds are chemical compounds formed the element sulfur (S). Common oxidation states of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except the noble gases.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols. An exception is the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.
Sulfolane (also tetramethylene sulfone, systematic name: 1λ6-thiolane-1,1-dione) is an organosulfur compound, formally a cyclic sulfone, with the formula (CH2)4SO2. It is a colorless liquid commonly used in the chemical industry as a solvent for extractive distillation and chemical reactions. Sulfolane was originally developed by the Shell Oil Company in the 1960s as a solvent to purify butadiene. Sulfolane is a polar aprotic solvent, and it is miscible with water.
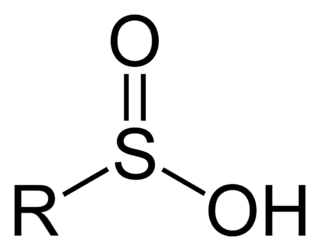
Sulfinic acids are oxoacids of sulfur with the structure RSO(OH). In these organosulfur compounds, sulfur is pyramidal.
In inorganic chemistry, sulfonyl halide groups occur when a sulfonyl functional group is singly bonded to a halogen atom. They have the general formula RSO2X, where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series.

In chemistry, a sulfenic acid is an organosulfur compound and oxoacid with the general formula R−S−OH. It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids and sulfonic acids, respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide.

Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source of butadiene.
Organotellurium chemistry describes the synthesis and properties of organotellurium compounds, chemical compounds containing a carbon-tellurium chemical bond. Organotellurium chemistry is a lightly studied area, in part because of it having few applications.
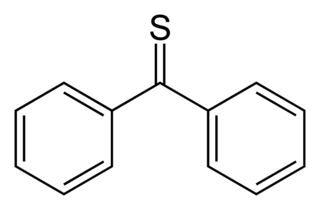
Thiobenzophenone is an organosulfur compound with the formula (C6H5)2CS. It is the prototypical thioketone. Unlike other thioketones that tend to dimerize to form rings and polymers, thiobenzophenone is quite stable, although it photoxidizes in air back to benzophenone and sulfur. Thiobenzophenone is deep blue and dissolves readily in many organic solvents.

Sulfinyl halide have the general formula R−S(O)−X, where X is a halogen. They are intermediate in oxidation level between sulfenyl halides, R−S−X, and sulfonyl halides, R−SO2−X. The best known examples are sulfinyl chlorides, thermolabile, moisture-sensitive compounds, which are useful intermediates for preparation of other sufinyl derivatives such as sulfinamides, sulfinates, sulfoxides, and thiosulfinates. Unlike the sulfur atom in sulfonyl halides and sulfenyl halides, the sulfur atom in sulfinyl halides is chiral, as shown for methanesulfinyl chloride.

Hydroxylamine-O-sulfonic acid (HOSA) or aminosulfuric acid is the inorganic compound with molecular formula H3NO4S that is formed by the sulfonation of hydroxylamine with oleum. It is a white, water-soluble and hygroscopic, solid, commonly represented by the condensed structural formula H2NOSO3H, though it actually exists as a zwitterion and thus is more accurately represented as +H3NOSO3−. It is used as a reagent for the introduction of amine groups (–NH2), for the conversion of aldehydes into nitriles and alicyclic ketones into lactams (cyclic amides), and for the synthesis of variety of nitrogen-containing heterocycles.

Diphenyl sulfide is an organosulfur compound with the chemical formula (C6H5)2S, often abbreviated as Ph2S, where Ph stands for phenyl. It is a colorless liquid with an unpleasant odor. Diphenyl sulfide is an aromatic sulfide. The molecule consists of two phenyl groups attached to a sulfur atom.



















