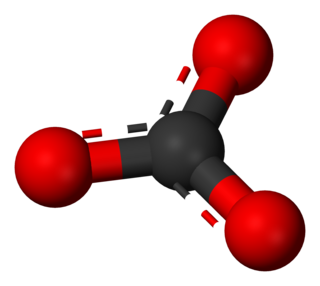
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula CO2−3. The word carbonate may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2.
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae.

A coordination complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.

Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry.

Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.

A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zero. The term molecule may or may not be used to refer to a polyatomic ion, depending on the definition used. The prefix poly- carries the meaning "many" in Greek, but even ions of two atoms are commonly described as polyatomic.
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge.
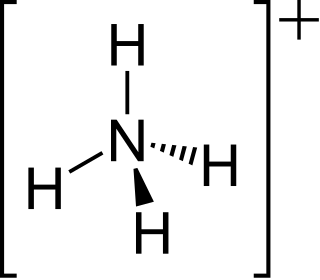
The ammonium cation is a positively-charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia. Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic groups.

In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group. In organic chemistry, the acyl group is usually derived from a carboxylic acid, in which case it has the formula RCO−, where R represents an alkyl group that is linked to the carbon atom of the group by a single bond. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acids and phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond.

The sulfate or sulphate ion is a polyatomic anion with the empirical formula SO2−4. Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid.
In chemistry, a hydride is formally the anion of hydrogen (H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.

In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions. These can be simple ions such as the sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic species such as the ammonium (NH+
4) and carbonate (CO2−
3) ions in ammonium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbours, so are not considered to be part of molecules, but instead part of a continuous three-dimensional network. Ionic compounds usually form crystalline structures when solid.
In chemistry, the valence or valency of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
In chemistry an antimonate is a compound which contains a metallic element, oxygen, and antimony in an oxidation state of +5. These compounds adopt polymeric structures with M-O-Sb linkages. They can be considered to be derivatives of the hypothetical antimonic acid H3SbO4, or combinations of metal oxides and antimony pentoxide, Sb2O5.
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC).
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce the H+ cation and the anion of the acid.
Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005 is the 2005 version of Nomenclature of Inorganic Chemistry. It is a collection of rules for naming inorganic compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC).

An ion is an atom or molecule with a net electrical charge.

A chemical compound is a chemical substance composed of many identical molecules containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed.











