
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon.

Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant medication fluoxetine (Prozac) and the material PTFE (Teflon). Elemental fluorine is produced from it. It is commonly used to etch glass and silicon wafers.

Tungsten(VI) fluoride, also known as tungsten hexafluoride, is an inorganic compound with the formula WF6. It is a toxic, corrosive, colorless gas, with a density of about 13 kg/m3 (22 lb/cu yd). It is one of the densest known gases under standard conditions. WF6 is commonly used by the semiconductor industry to form tungsten films, through the process of chemical vapor deposition. This layer is used in a low-resistivity metallic "interconnect". It is one of seventeen known binary hexafluorides.

Magnesium fluoride is an ionically bonded inorganic compound with the formula MgF2. The compound is a white crystalline salt and is transparent over a wide range of wavelengths, with commercial uses in optics that are also used in space telescopes. It occurs naturally as the rare mineral sellaite.

Sodium fluoride (NaF) is an inorganic compound with the formula NaF. It is a colorless or white solid that is readily soluble in water. It is used in trace amounts in the fluoridation of drinking water to prevent tooth decay, and in toothpastes and topical pharmaceuticals for the same purpose. In 2020, it was the 265th most commonly prescribed medication in the United States, with more than 1 million prescriptions. It is also used in metallurgy and in medical imaging.

Ammonium fluoride is the inorganic compound with the formula NH4F. It crystallizes as small colourless prisms, having a sharp saline taste, and is highly soluble in water. Like all fluoride salts, it is moderately toxic in both acute and chronic overdose.

FLiNaK is the name of the ternary eutectic alkaline metal fluoride salt mixture LiF-NaF-KF (46.5-11.5-42 mol %). It has a melting point of 454 °C and a boiling point of 1570 °C. It is used as electrolyte for the electroplating of refractory metals and compounds like titanium, tantalum, hafnium, zirconium and their borides. FLiNaK also could see potential use as a coolant in the very high temperature reactor, a type of nuclear reactor.

Lithium fluoride is an inorganic compound with the chemical formula LiF. It is a colorless solid that transitions to white with decreasing crystal size. Although odorless, lithium fluoride has a bitter-saline taste. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten salts. Partly because Li and F are both light elements, and partly because F2 is highly reactive, formation of LiF from the elements releases one of the highest energies per mass of reactants, second only to that of BeO.

Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by researchers, unlike the related iron(III) chloride. Anhydrous iron(III) fluoride is white, whereas the hydrated forms are light pink.

Hydrogen fluoride (fluorane) is an inorganic compound with chemical formula HF. It is a very poisonous, colorless gas or liquid that dissolves in water to yield an aqueous solution termed hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils at near room temperature, much higher than other hydrogen halides.

Strontium fluoride, SrF2, also called strontium difluoride and strontium(II) fluoride, is a fluoride of strontium. It is a brittle white crystalline solid. In nature, it appears as the very rare mineral strontiofluorite.

Hexafluorosilicic acid is an inorganic compound with the chemical formula H
2SiF
6. Aqueous solutions of hexafluorosilicic acid consist of salts of the cation and hexafluorosilicate anion. These salts and their aqueous solutions are colorless.

Ammonium bifluoride is the inorganic compound with the formula [NH4][HF2] or [NH4]F·HF. It is produced from ammonia and hydrogen fluoride. This colourless salt is a glass-etchant and an intermediate in a once-contemplated route to hydrofluoric acid.

Aluminium fluoride is an inorganic compound with the formula AlF3. It forms hydrates AlF3·xH2O. Anhydrous AlF3 and its hydrates are all colorless solids. Anhydrous AlF3 is used in the production of aluminium metal. Several occur as minerals.
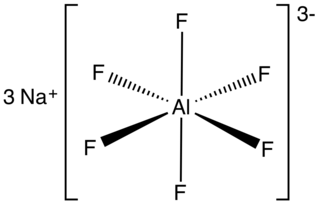
Sodium hexafluoroaluminate is an inorganic compound with formula Na3AlF6. This white solid, discovered in 1799 by Peder Christian Abildgaard (1740–1801), occurs naturally as the mineral cryolite and is used extensively in the industrial production of aluminium metal. The compound is the sodium (Na+) salt of the hexafluoroaluminate (AlF63−) ion.
The bifluoride ion is an inorganic anion with the chemical formula [HF2]−. The anion is colorless. Salts of bifluoride are commonly encountered in the reactions of fluoride salts with hydrofluoric acid. The commercial production of fluorine involves electrolysis of bifluoride salts.

Potassium bifluoride is the inorganic compound with the formula K[HF2]. This colourless salt consists of the potassium cation and the bifluoride anion. The salt is used as an etchant for glass. Sodium bifluoride is related and is also of commercial use as an etchant as well as in cleaning products.
A monofluoride is a chemical compound with one fluoride per formula unit. For a binary compound, this is the formula XF.
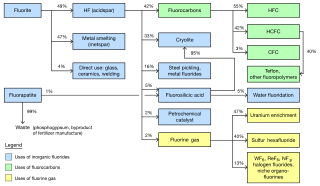
The global market for chemicals from fluorine was about US$16 billion per year as of 2006. The industry was predicted to reach 2.6 million metric tons per year by 2015. The largest market is the United States. Western Europe is the second largest. Asia Pacific is the fastest growing region of production. China in particular has experienced significant growth as a fluorochemical market and is becoming a producer of them as well. Fluorite mining was estimated in 2003 to be a $550 million industry, extracting 4.5 million tons per year.
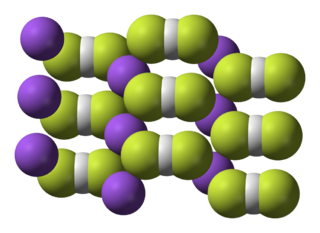
Sodium bifluoride is the inorganic compound with the formula Na[HF2]. It is a salt of sodium cation and bifluoride anion. It is a white, water-soluble solid that decomposes upon heating. Sodium bifluoride is non-flammable, hygroscopic, and has a pungent smell. Sodium bifluoride has a number of applications in industry.





















