In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.

Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2).

Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:

Chlorine pentafluoride is an interhalogen compound with formula ClF5. This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its high-resolution 19F NMR spectrum. It was first synthesized in 1963.

Nickel(II) fluoride is the chemical compound with the formula NiF2. It is an ionic compound of nickel and fluorine and forms yellowish to green tetragonal crystals. Unlike many fluorides, NiF2 is stable in air.
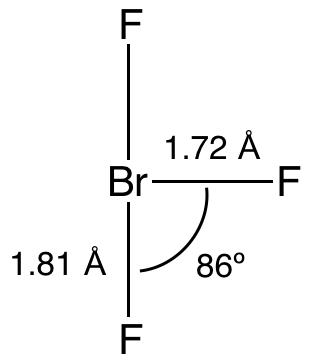
Bromine trifluoride is an interhalogen compound with the formula BrF3. At room temperature, it is a straw-coloured liquid with a pungent odor which decomposes violently on contact with water and organic compounds. It is a powerful fluorinating agent and an ionizing inorganic solvent. It is used to produce uranium hexafluoride (UF6) in the processing and reprocessing of nuclear fuel.

Bromine pentafluoride, BrF5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent.

Nitrogen trifluoride is an inorganic, colorless, non-flammable, toxic gas with a slightly musty odor. It finds increasing use within the manufacturing of flat-panel displays, photovoltaics, LEDs and other microelectronics. Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gas. Its atmospheric burden exceeded 2 parts per trillion during 2019 and has doubled every five years since the late 20th century.

Cobalt(III) fluoride is the inorganic compound with the formula CoF3. Hydrates are also known. The anhydrous compound is a hygroscopic brown solid. It is used to synthesize organofluorine compounds.

Silver(II) fluoride is a chemical compound with the formula AgF2. It is a rare example of a silver(II) compound - silver usually exists in its +1 oxidation state. It is used as a fluorinating agent.
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds.

Sulfur tetrafluoride is the chemical compound with the formula SF4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.

Aluminium fluoride is an inorganic compound with the formula AlF3. It forms hydrates AlF3·xH2O. Anhydrous AlF3 and its hydrates are all colorless solids. Anhydrous AlF3 is used in the production of aluminium. Several occur as minerals.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.
Perchloryl fluoride is a reactive gas with the chemical formula ClO
3F. It has a characteristic sweet odor that resembles gasoline and kerosene. It is toxic and is a powerful oxidizing and fluorinating agent. It is the acid fluoride of perchloric acid.

Antimony trifluoride is the inorganic compound with the formula SbF3. Sometimes called Swarts' reagent, it is one of two principal fluorides of antimony, the other being SbF5. It appears as a white solid. As well as some industrial applications, it is used as a reagent in inorganic and organofluorine chemistry.
Arsenic trifluoride is a chemical compound of arsenic and fluorine with the chemical formula AsF3. It is a colorless liquid which reacts readily with water.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.

Promethium(III) fluoride or promethium trifluoride is a salt of promethium and fluorine with the formula PmF3.

Terbium(IV) fluoride is an inorganic compound with a chemical formula TbF4. It is a white solid that is a strong oxidizer. It is also a strong fluorinating agent, emitting relatively pure atomic fluorine when heated, rather than the mixture of fluoride vapors emitted from cobalt(III) fluoride or cerium(IV) fluoride. It can be produced by the reaction between very pure terbium(III) fluoride and xenon difluoride, chlorine trifluoride or fluorine gas:




















