An analgesic drug, also called simply an analgesic, pain reliever, or painkiller, is any member of the group of drugs used for pain management. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects.

Hydrocodone, also known as dihydrocodeinone, is a semisynthetic opioid used to treat pain and as a cough suppressant. It is taken by mouth. Typically it is dispensed as the combination acetaminophen/hydrocodone or ibuprofen/hydrocodone for pain severe enough to require an opioid and in combination with homatropine methylbromide to relieve cough. It is also available by itself in a long-acting form under the brand name Zohydro ER, among others, to treat severe pain of a prolonged duration. Hydrocodone is a controlled drug: in the United States a Schedule II Controlled Substance.

Methadone, sold under the brand names Dolophine and Methadose among others, is a synthetic opioid agonist used for chronic pain and also for opioid use disorder. It is used to treat chronic pain, and it is also used to treat addiction to heroin or other opioids. Prescribed for daily use, the medicine relieves cravings and removes withdrawal symptoms. Withdrawal management using methadone can be accomplished in less than a month, or it may be done gradually over a longer period of time, or simply maintained for the rest of the patient's life. While a single dose has a rapid effect, maximum effect can take up to five days of use. After long-term use, in people with normal liver function, effects last 8 to 36 hours. Methadone is usually taken by mouth and rarely by injection into a muscle or vein.

Serotonin syndrome (SS) is a group of symptoms that may occur with the use of certain serotonergic medications or drugs. The symptoms can range from mild to severe, and are potentially fatal. Symptoms in mild cases include high blood pressure and a fast heart rate; usually without a fever. Symptoms in moderate cases include high body temperature, agitation, increased reflexes, tremor, sweating, dilated pupils, and diarrhea. In severe cases, body temperature can increase to greater than 41.1 °C (106.0 °F). Complications may include seizures and extensive muscle breakdown.

Tramadol, sold under the brand name Ultram among others, is an opioid pain medication and a serotonin–norepinephrine reuptake inhibitor (SNRI) used to treat moderately severe pain. When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an hour. It is also available by injection. It is available in combination with paracetamol (acetaminophen).

Hydromorphone, also known as dihydromorphinone, and sold under the brand name Dilaudid among others, is a morphinan opioid used to treat moderate to severe pain. Typically, long-term use is only recommended for pain due to cancer. It may be used by mouth or by injection into a vein, muscle, or under the skin. Effects generally begin within half an hour and last for up to five hours. A 2016 Cochrane review found little difference in benefit between hydromorphone and other opioids for cancer pain.

Opioids are a class of drugs that derive from, or mimic, natural substances found in the opium poppy plant. Opioids work in the brain to produce a variety of effects, including pain relief. As a class of substances, they act on opioid receptors to produce morphine-like effects.
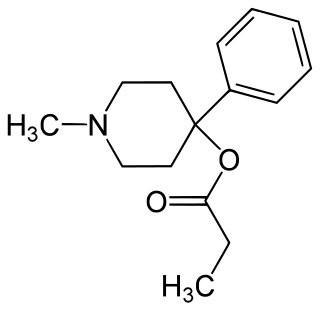
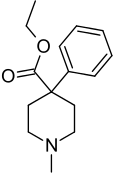
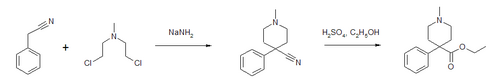
Desmethylprodine or 1-methyl-4-phenyl-4-propionoxypiperidine is an opioid analgesic drug developed in the 1940s by researchers at Hoffmann-La Roche. Desmethylprodine has been labeled by the DEA as a Schedule I drug in the United States. It is an analog of pethidine (meperidine) a Schedule II drug. Chemically, it is a reversed ester of pethidine which has about 70% of the potency of morphine. Unlike its derivative prodine, it was not reported to exhibit optical isomerism. It was reported to have 30 times the activity of pethidine and a greater analgesic effect than morphine in rats, and it was demonstrated to cause central nervous system stimulation in mice.

Buprenorphine, sold under the brand name Subutex among others, is an opioid used to treat opioid use disorder, acute pain, and chronic pain. It can be used under the tongue (sublingual), in the cheek (buccal), by injection, as a skin patch (transdermal), or as an implant. For opioid use disorder, the patient must have moderate opioid withdrawal symptoms before buprenorphine can be administered under direct observation of a health-care provider.

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.

Phenoperidine, is an opioid analgesic which is structurally related to pethidine and is used clinically as a general anesthetic.

Hydroxypethidine (Bemidone) is an opioid analgesic that is an analogue of the more commonly used pethidine (meperidine). Hydroxypethidine is slightly more potent than meperidine as an analgesic, 1.5x meperidine in potency, and it also has NMDA antagonist properties like its close relative ketobemidone.

Desmetramadol, also known as O-desmethyltramadol (O-DSMT), is an opioid analgesic and the main active metabolite of tramadol. Tramadol is demethylated by the liver enzyme CYP2D6 to desmetramadol in the same way as codeine, and so similarly to the variation in effects seen with codeine, individuals who have a less active form of CYP2D6 will tend to have reduced analgesic effects from tramadol. Because desmetramadol itself does not need to be metabolized to induce an analgesic effect, it can be used in individuals with low CYP2D6 activity unlike tramadol.
Tapentadol, brand names Nucynta among others, is a centrally acting opioid analgesic of the benzenoid class with a dual mode of action as an agonist of the μ-opioid receptor and as a norepinephrine reuptake inhibitor (NRI). Analgesia occurs within 32 minutes of oral administration, and lasts for 4–6 hours.
Dezocine, sold under the brand name Dalgan, is an atypical opioid analgesic which is used in the treatment of pain. It is used by intravenous infusion and intramuscular injection.

Prodine is an opioid analgesic that is an analog of pethidine (meperidine). It was developed in Germany in the late 1940s.
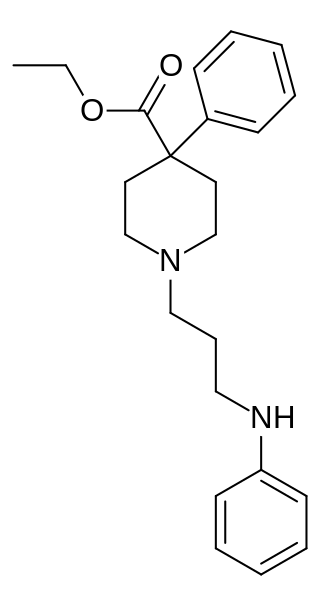
Piminodine (Alvodine) is an opioid analgesic that is an analogue of pethidine (meperidine). It was used in medicine briefly during the 1960s and 70s, but has largely fallen out of clinical use. It was used particularly for obstetric analgesia and in dental procedures and, like pethidine, could be combined with hydroxyzine to intensify the effects. The duration of action is 2–4 hours; 7.5–10 mg via the subcutaneous route is the most common starting dose, being equal to 80–100 mg of pethidine, 40–60 mg of alphaprodine and 10 mg of morphine. Oral formulations were also available.

Nefopam, sold under the brand name Acupan among others, is a centrally acting, non-opioid painkilling medication, that is primarily used to treat moderate to severe pain.
An equianalgesic chart is a conversion chart that lists equivalent doses of analgesics. Equianalgesic charts are used for calculation of an equivalent dose between different analgesics. Tables of this general type are also available for NSAIDs, benzodiazepines, depressants, stimulants, anticholinergics and others.

An opiate is an alkaloid substance derived from opium. It differs from the similar term opioid in that the latter is used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant Papaver somniferum. The psychoactive compounds found in the opium plant include morphine, codeine, and thebaine. Opiates have long been used for a variety of medical conditions, with evidence of opiate trade and use for pain relief as early as the eighth century AD. Most opiates are considered drugs with moderate to high abuse potential and are listed on various "Substance-Control Schedules" under the Uniform Controlled Substances Act of the United States of America.