
Apocynaceae is a family of flowering plants that includes trees, shrubs, herbs, stem succulents, and vines, commonly known as the dogbane family, because some taxa were used as dog poison. Members of the family are native to the European, Asian, African, Australian, and American tropics or subtropics, with some temperate members. The former family Asclepiadaceae is considered a subfamily of Apocynaceae and contains 348 genera. A list of Apocynaceae genera may be found here.

Vinca is a genus of flowering plants in the family Apocynaceae, native to Europe, northwest Africa and southwest Asia. The English name periwinkle is shared with the related genus Catharanthus.

Vinblastine (VBL), sold under the brand name Velban among others, is a chemotherapy medication, typically used with other medications, to treat a number of types of cancer. This includes Hodgkin's lymphoma, non-small-cell lung cancer, bladder cancer, brain cancer, melanoma, and testicular cancer. It is given by injection into a vein.

Catharanthus roseus, commonly known as bright eyes, Cape periwinkle, graveyard plant, Madagascar periwinkle, old maid, pink periwinkle, rose periwinkle, is a perennial species of flowering plant in the family Apocynaceae. It is native and endemic to Madagascar, but is grown elsewhere as an ornamental and medicinal plant, and now has a pantropical distribution. It is a source of the drugs vincristine and vinblastine, used to treat cancer. It was formerly included in the genus Vinca as Vinca rosea.
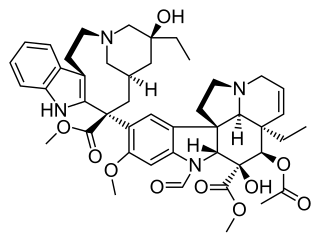
Vinca alkaloids are a set of anti-mitotic and anti-microtubule alkaloid agents originally derived from the periwinkle plant Catharanthus roseus and other vinca plants. They block beta-tubulin polymerization in a dividing cell.

Voacangine is an alkaloid found predominantly in the root bark of the Voacanga africana tree, as well as in other plants such as Tabernanthe iboga, Tabernaemontana africana, Trachelospermum jasminoides, Tabernaemontana divaricata and Ervatamia yunnanensis. It is an iboga alkaloid which commonly serves as a precursor for the semi-synthesis of ibogaine. It has been demonstrated in animals to have similar anti-addictive properties to ibogaine itself. It also potentiates the effects of barbiturates. Under UV-A and UV-B light its crystals fluoresce blue-green, and it is soluble in ethanol.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.

Iridoids are a type of monoterpenoids in the general form of cyclopentanopyran, found in a wide variety of plants and some animals. They are biosynthetically derived from 8-oxogeranial. Iridoids are typically found in plants as glycosides, most often bound to glucose.
Strictosidine synthase (EC 4.3.3.2) is an enzyme in alkaloid biosynthesis that catalyses the condensation of tryptamine with secologanin to form strictosidine in a formal Pictet–Spengler reaction:

Akuammine (vincamajoridine) is an indole alkaloid. It is the most abundant alkaloid found in the seeds from the tree Picralima nitida, commonly known as akuamma, comprising 0.56% of the dried powder. It has also been isolated from Vinca major. Akuammine is structurally related to yohimbine, mitragynine and more distantly Voacangine, all of which are alkaloid plant products with pharmacological properties.

Ajmalicine, also known as δ-yohimbine or raubasine, is an antihypertensive drug used in the treatment of high blood pressure. It has been marketed under numerous brand names including Card-Lamuran, Circolene, Cristanyl, Duxil, Duxor, Hydroxysarpon, Iskedyl, Isosarpan, Isquebral, Lamuran, Melanex, Raunatin, Saltucin Co, Salvalion, and Sarpan. It is an alkaloid found naturally in various plants such as Rauvolfia spp., Catharanthus roseus, and Mitragyna speciosa.
3α(S)-strictosidine β-glucosidase (EC 3.2.1.105) is an enzyme with systematic name strictosidine β-D-glucohydrolase. It catalyses the following chemical reaction:

Catharanthine is a terpene indole alkaloid produced by the medicinal plant Catharanthus roseus and Tabernaemontana divaricata. Catharanthine is derived from strictosidine, but the exact mechanism by which this happens is currently unknown. Catharanthine is one of the two precursors that form vinblastine, the other being vindoline.

Strictosidine is a natural chemical compound and is classified as a glucoalkaloid and a vinca alkaloid. It is formed by the Pictet–Spengler condensation reaction of tryptamine with secologanin, catalyzed by the enzyme strictosidine synthase. Thousands of strictosidine derivatives are sometimes referred to by the broad phrase of monoterpene indole alkaloids. Strictosidine is an intermediate in the biosynthesis of numerous pharmaceutically valuable metabolites including quinine, camptothecin, ajmalicine, serpentine, vinblastine, vincristine and mitragynine.

3-Hydroxy-16-methoxy-2,3-dihydrotabersonine is a terpene indole alkaloid produced by Catharanthus roseus. The metabolite is a substrate for 3-hydroxy-16-methoxy-2,3-dihydrotabersonine N-methyltransferase (NMT) which transfers a methyl group to the nitrogen of the indole ring forming desacetoxyvindoline. The enzyme catalyzing the formation of 3-hydroxy-16-methoxy-2,3-dihydrotabersonine from 16-methoxytabersonine is currently unknown, but is a result of hydration of the double bond connecting the 6 and 13 position carbons.

Stemmadenine is a terpene indole alkaloid. Stemmadenine is believed to be formed from preakuammicine by a carbon-carbon bond cleavage. Cleavage of a second carbon-carbon bond is thought to form dehydrosecodine. The enzymes forming stemmadenine and using it as a substrate remain unknown to date. It is thought to be intermediate compound in many different biosynthetic pathways such as in Aspidosperma species. Many alkaloids are proposed to be produced through intermediate stemmadenine. Some of them are:

Tabernaemontanine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.

Dregamine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Ervatamia hirta and Tabernaemontana divaricata.

Vobasine is a naturally occurring monoterpene indole alkaloid found in several species in the genus Tabernaemontana including Tabernaemontana divaricata.
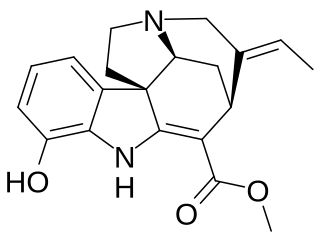
Vinervine is a monoterpene indole alkaloid of the Vinca sub-group. It is a derivative of akuammicine, with one additional hydroxy (OH) group in the indole portion, hence it is also known as 12-hydroxyakuammicine.




















