
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.
Inorganic ions in animals and plants are ions necessary for vital cellular activity. In body tissues, ions are also known as electrolytes, essential for the electrical activity needed to support muscle contractions and neuron activation. They contribute to osmotic pressure of body fluids as well as performing a number of other important functions. Below is a list of some of the most important ions for living things as well as examples of their functions:

Potassium channels are the most widely distributed type of ion channel found in virtually all organisms. They form potassium-selective pores that span cell membranes. Potassium channels are found in most cell types and control a wide variety of cell functions.
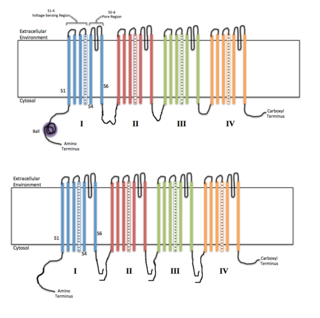
Voltage-gated ion channels are a class of transmembrane proteins that form ion channels that are activated by changes in the electrical membrane potential near the channel. The membrane potential alters the conformation of the channel proteins, regulating their opening and closing. Cell membranes are generally impermeable to ions, thus they must diffuse through the membrane through transmembrane protein channels. They have a crucial role in excitable cells such as neuronal and muscle tissues, allowing a rapid and co-ordinated depolarization in response to triggering voltage change. Found along the axon and at the synapse, voltage-gated ion channels directionally propagate electrical signals. Voltage-gated ion-channels are usually ion-specific, and channels specific to sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) ions have been identified. The opening and closing of the channels are triggered by changing ion concentration, and hence charge gradient, between the sides of the cell membrane.
Voltage-gated calcium channels (VGCCs), also known as voltage-dependent calcium channels (VDCCs), are a group of voltage-gated ion channels found in the membrane of excitable cells (e.g., muscle, glial cells, neurons, etc.) with a permeability to the calcium ion Ca2+. These channels are slightly permeable to sodium ions, so they are also called Ca2+–Na+ channels, but their permeability to calcium is about 1000-fold greater than to sodium under normal physiological conditions.

G protein-gated ion channels are a family of transmembrane ion channels in neurons and atrial myocytes that are directly gated by G proteins.
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's membrane. They belong to the superfamily of cation channels.
Calcium-activated potassium channels are potassium channels gated by calcium, or that are structurally or phylogenetically related to calcium gated channels. They were first discovered in 1958 by Gardos who saw that calcium levels inside of a cell could affect the permeability of potassium through that cell membrane. Then in 1970, Meech was the first to observe that intracellular calcium could trigger potassium currents. In humans they are divided into three subtypes: large conductance or BK channels, which have very high conductance which range from 100 to 300 pS, intermediate conductance or IK channels, with intermediate conductance ranging from 25 to 100 pS, and small conductance or SK channels with small conductances from 2-25 pS.

Voltage-gated potassium channels (VGKCs) are transmembrane channels specific for potassium and sensitive to voltage changes in the cell's membrane potential. During action potentials, they play a crucial role in returning the depolarized cell to a resting state.
Two-pore channels (TPCs) are eukaryotic intracellular voltage-gated and ligand gated cation selective ion channels. There are two known paralogs in the human genome, TPC1s and TPC2s. In humans, TPC1s are sodium selective and TPC2s conduct sodium ions, calcium ions and possibly hydrogen ions. Plant TPC1s are non-selective channels. Expression of TPCs are found in both plant vacuoles and animal acidic organelles. These organelles consist of endosomes and lysosomes. TPCs are formed from two transmembrane non-equivalent tandem Shaker-like, pore-forming subunits, dimerized to form quasi-tetramers. Quasi-tetramers appear very similar to tetramers, but are not quite the same. Some key roles of TPCs include calcium dependent responses in muscle contraction(s), hormone secretion, fertilization, and differentiation. Disorders linked to TPCs include membrane trafficking, Parkinson's disease, Ebola, and fatty liver.

Potassium voltage-gated channel subfamily E member 1 is a protein that in humans is encoded by the KCNE1 gene.
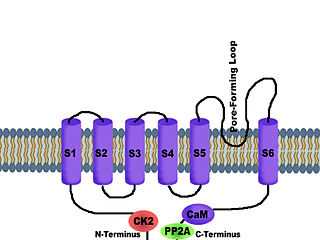
SK channels are a subfamily of calcium-activated potassium channels. They are so called because of their small single channel conductance in the order of 10 pS. SK channels are a type of ion channel allowing potassium cations to cross the cell membrane and are activated (opened) by an increase in the concentration of intracellular calcium through N-type calcium channels. Their activation limits the firing frequency of action potentials and is important for regulating afterhyperpolarization in the neurons of the central nervous system as well as many other types of electrically excitable cells. This is accomplished through the hyperpolarizing leak of positively charged potassium ions along their concentration gradient into the extracellular space. This hyperpolarization causes the membrane potential to become more negative. SK channels are thought to be involved in synaptic plasticity and therefore play important roles in learning and memory.
T-type calcium channels are low voltage activated calcium channels that become inactivated during cell membrane hyperpolarization but then open to depolarization. The entry of calcium into various cells has many different physiological responses associated with it. Within cardiac muscle cell and smooth muscle cells voltage-gated calcium channel activation initiates contraction directly by allowing the cytosolic concentration to increase. Not only are T-type calcium channels known to be present within cardiac and smooth muscle, but they also are present in many neuronal cells within the central nervous system. Different experimental studies within the 1970s allowed for the distinction of T-type calcium channels from the already well-known L-type calcium channels. The new T-type channels were much different from the L-type calcium channels due to their ability to be activated by more negative membrane potentials, had small single channel conductance, and also were unresponsive to calcium antagonist drugs that were present. These distinct calcium channels are generally located within the brain, peripheral nervous system, heart, smooth muscle, bone, and endocrine system.

The L-type calcium channel is part of the high-voltage activated family of voltage-dependent calcium channel. "L" stands for long-lasting referring to the length of activation. This channel has four isoforms: Cav1.1, Cav1.2, Cav1.3, and Cav1.4.

K+ channel tetramerisation domain is the N-terminal, cytoplasmic tetramerisation domain (T1) of voltage-gated K+ channels. It defines molecular determinants for subfamily-specific assembly of alpha-subunits into functional tetrameric channels. It is distantly related to the BTB/POZ domain Pfam PF00651.
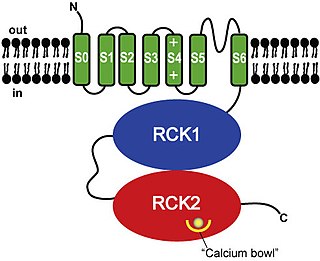
Calcium-activated potassium channel subunit alpha-1 also known as large conductance calcium-activated potassium channel, subfamily M, alpha member 1 (KCa1.1), or BK channel alpha subunit, is a voltage gated potassium channel encoded by the KCNMA1 gene and characterized by their large conductance of potassium ions (K+) through cell membranes.

Calcium-activated potassium channel subunit beta-1 is a protein that in humans is encoded by the KCNMB1 gene.

Potassium voltage-gated channel, Shab-related subfamily, member 1, also known as KCNB1 or Kv2.1, is a protein that, in humans, is encoded by the KCNB1 gene.
Calcium-activated potassium channel subunit beta-2 is a protein that in humans is encoded by the KCNMB2 gene.

In neuroscience, ball and chain inactivation is a model to explain the fast inactivation mechanism of voltage-gated ion channels. The process is also called hinged-lid inactivation or N-type inactivation. A voltage-gated ion channel can be in three states: open, closed, or inactivated. The inactivated state is mainly achieved through fast inactivation, by which a channel transitions rapidly from an open to an inactivated state. The model proposes that the inactivated state, which is stable and non-conducting, is caused by the physical blockage of the pore. The blockage is caused by a "ball" of amino acids connected to the main protein by a string of residues on the cytoplasmic side of the membrane. The ball enters the open channel and binds to the hydrophobic inner vestibule within the channel. This blockage causes inactivation of the channel by stopping the flow of ions. This phenomenon has mainly been studied in potassium channels and sodium channels.














