Related Research Articles

Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.
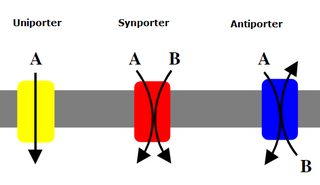
Uniporters, also known as solute carriers or facilitated transporters, are a type of membrane transport protein that passively transports solutes across a cell membrane. It uses facilitated diffusion for the movement of solutes down their concentration gradient from an area of high concentration to an area of low concentration. Unlike active transport, it does not require energy in the form of ATP to function. Uniporters are specialized to carry one specific ion or molecule and can be categorized as either channels or carriers. Facilitated diffusion may occur through three mechanisms: uniport, symport, or antiport. The difference between each mechanism depends on the direction of transport, in which uniport is the only transport not coupled to the transport of another solute.
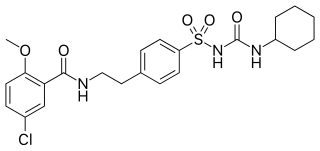
Glibenclamide, also known as glyburide, is an antidiabetic medication used to treat type 2 diabetes. It is recommended that it be taken together with diet and exercise. It may be used with other antidiabetic medication. It is not recommended for use by itself in type 1 diabetes. It is taken by mouth.

The renal outer medullary potassium channel (ROMK) is an ATP-dependent potassium channel (Kir1.1) that transports potassium out of cells. It plays an important role in potassium recycling in the thick ascending limb (TAL) and potassium secretion in the cortical collecting duct (CCD) of the nephron. In humans, ROMK is encoded by the KCNJ1 gene. Multiple transcript variants encoding different isoforms have been found for this gene.
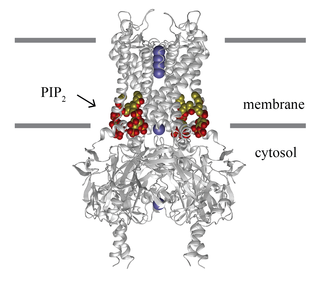
Inward-rectifier potassium channels (Kir, IRK) are a specific lipid-gated subset of potassium channels. To date, seven subfamilies have been identified in various mammalian cell types, plants, and bacteria. They are activated by phosphatidylinositol 4,5-bisphosphate (PIP2). The malfunction of the channels has been implicated in several diseases. IRK channels possess a pore domain, homologous to that of voltage-gated ion channels, and flanking transmembrane segments (TMSs). They may exist in the membrane as homo- or heterooligomers and each monomer possesses between 2 and 4 TMSs. In terms of function, these proteins transport potassium (K+), with a greater tendency for K+ uptake than K+ export. The process of inward-rectification was discovered by Denis Noble in cardiac muscle cells in 1960s and by Richard Adrian and Alan Hodgkin in 1970 in skeletal muscle cells.
In molecular biology, the sulfonylurea receptors (SUR) are membrane proteins which are the molecular targets of the sulfonylurea class of antidiabetic drugs whose mechanism of action is to promote insulin release from pancreatic beta cells. More specifically, SUR proteins are subunits of the inward-rectifier potassium ion channels Kir6.x. The association of four Kir6.x and four SUR subunits form an ion conducting channel commonly referred to as the KATP channel.

Kir6.2 is a major subunit of the ATP-sensitive K+ channel, a lipid-gated inward-rectifier potassium ion channel. The gene encoding the channel is called KCNJ11 and mutations in this gene are associated with congenital hyperinsulinism.

Protein kinase C epsilon type (PKCε) is an enzyme that in humans is encoded by the PRKCE gene. PKCε is an isoform of the large PKC family of protein kinases that play many roles in different tissues. In cardiac muscle cells, PKCε regulates muscle contraction through its actions at sarcomeric proteins, and PKCε modulates cardiac cell metabolism through its actions at mitochondria. PKCε is clinically significant in that it is a central player in cardioprotection against ischemic injury and in the development of cardiac hypertrophy.

ATP-binding cassette transporter sub-family C member 8 is a protein that in humans is encoded by the ABCC8 gene. ABCC8 orthologs have been identified in all mammals for which complete genome data are available.

G protein-activated inward rectifier potassium channel 2 is a protein that in humans is encoded by the KCNJ6 gene. Mutation in KCNJ6 gene has been proposed to be the cause of Keppen-Lubinsky Syndrome (KPLBS).

Potassium inwardly-rectifying channel, subfamily J, member 8, also known as KCNJ8, is a human gene encoding the Kir6.1 protein. A mutation in KCNJ8 has been associated with cardiac arrest in the early repolarization syndrome.

G protein-activated inward rectifier potassium channel 4(GIRK-4) is a protein that in humans is encoded by the KCNJ5 gene and is a type of G protein-gated ion channel.

Potassium voltage-gated channel, Isk-related family, member 3 (KCNE3), also known as MinK-related peptide 2(MiRP2) is a protein that in humans is encoded by the KCNE3 gene.
Potassium voltage-gated channel subfamily D member 3 also known as Kv4.3 is a protein that in humans is encoded by the KCND3 gene. It contributes to the cardiac transient outward potassium current (Ito1), the main contributing current to the repolarizing phase 1 of the cardiac action potential.

Alpha-endosulfine is a protein that in humans is encoded by the ENSA gene.

ATP-binding cassette, sub-family C member 9 (ABCC9) also known as sulfonylurea receptor 2 (SUR2) is an ATP-binding cassette transporter that in humans is encoded by the ABCC9 gene.
HMR 1883 and its sodium salt HMR 1098, are experimental anti-arrhythmic drugs classified as sulfonylthiourea compounds. Their main purpose is to treat ventricular fibrillation caused by myocardial ischemia. They were synthesized via structural modifications to glibenclamide, an antidiabetic drug. Both HMR 1883 and glibenclamide act by inactivating the ATP-sensitive potassium channels (KATP) responsible for potassium efflux. Unlike glibenclamide, HMR 1883 has been suggested to target selectively the Kir6.2/SUR2A KATP subtype, found mostly in the membranes of cardiac cells. However, data showing that HMR 1098 inhibits the Kir6.2/SUR1 KATP subtype found in insulin-secreting pancreatic beta cells challenges this view.

Rottlerin (mallotoxin) is a polyphenol natural product isolated from the Asian tree Mallotus philippensis. Rottlerin displays a complex spectrum of pharmacology.
Diallyl trisulfide (DATS), also known as Allitridin, is an organosulfur compound with the formula S(SCH2CH=CH2)2. It is one of several compounds produced by hydrolysis of allicin, including diallyl disulfide and diallyl tetrasulfide; DATS is one of the most potent.

Colin G. Nichols FRS is the Carl Cori Endowed Professor, and Director of the Center for Investigation of Membrane Excitability Diseases at Washington University in St. Louis, Missouri.
References
- ↑ Stephan D, Winkler M, Kühner P, Russ U, Quast U (September 2006). "Selectivity of repaglinide and glibenclamide for the pancreatic over the cardiovascular K(ATP) channels". Diabetologia. 49 (9): 2039–48. doi: 10.1007/s00125-006-0307-3 . PMID 16865362.
- ↑ Babenko AP, Aguilar-Bryan L, Bryan J (1998). "A View of Sur/kir6.x, Katpchannels". Annual Review of Physiology. 60: 667–687. doi:10.1146/annurev.physiol.60.1.667. PMID 9558481.
- ↑ Noma A (1983). "ATP-regulated K+ channels in cardiac muscle". Nature. 305 (5930): 147–148. doi:10.1038/305147a0. PMID 6310409. S2CID 31679373.
- ↑ Ashcroft F (1984). "Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells". Nature. 312 (5993): 446–448. Bibcode:1984Natur.312..446A. doi:10.1038/312446a0. PMID 6095103. S2CID 4340710.
- ↑ Rorsman P, Ramracheya R, Rorsman N, Zhang Q (2014). "ATP-regulated potassium channels and voltage-gated calcium channels in pancreatic alpha and beta cells: similar functions but reciprocal effects on secretion". Diabetologia. 57 (9): 1749–1761. doi:10.1007/s00125-014-3279-8. PMID 24906950.
- ↑ Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J (November 1995). "Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor". Science. 270 (5239): 1166–70. doi:10.1126/science.270.5239.1166. PMID 7502040. S2CID 26409797.
- ↑ Seino S, Miki T (February 2003). "Physiological and pathophysiological roles of ATP-sensitive K+ channels". Prog. Biophys. Mol. Biol. 81 (2): 133–76. doi:10.1016/S0079-6107(02)00053-6. PMID 12565699.
- 1 2 3 4 Zhuo ML, Huang Y, Liu DP, Liang CC (April 2005). "KATP channel: relation with cell metabolism and role in the cardiovascular system". Int. J. Biochem. Cell Biol. 37 (4): 751–64. doi:10.1016/j.biocel.2004.10.008. PMID 15694835.
- ↑ Inoue I, Nagase H, Kishi K, Higuti T (July 1991). "ATP-sensitive K+ channel in the mitochondrial inner membrane". Nature. 352 (6332): 244–7. doi:10.1038/352244a0. PMID 1857420. S2CID 4358756.
- ↑ Lacza Z, Snipes JA, Miller AW, Szabó C, Grover G, Busija DW (November 2003). "Heart mitochondria contain functional ATP-dependent K+ channels". J. Mol. Cell. Cardiol. 35 (11): 1339–47. doi:10.1016/S0022-2828(03)00249-9. PMID 14596790.
- ↑ Mironova GD, Grigoriev SM, Skarga YuYu, Negoda AE, Kolomytkin OV (1997). "ATP-dependent potassium channel from rat liver mitochondria: inhibitory analysis, channel clusterization". Membr Cell Biol. 10 (5): 583–91. PMID 9225262.
- ↑ Ardehali H, Chen Z, Ko Y, Mejía-Alvarez R, Marbán E (August 2004). "Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity". Proc. Natl. Acad. Sci. U.S.A. 101 (32): 11880–5. doi: 10.1073/pnas.0401703101 . PMC 511068 . PMID 15284438.
- ↑ Quesada I, Rovira JM, Martin F, Roche E, Nadal A, Soria B (July 2002). "Nuclear KATP channels trigger nuclear Ca(2+) transients that modulate nuclear function". Proc. Natl. Acad. Sci. U.S.A. 99 (14): 9544–9. doi: 10.1073/pnas.142039299 . PMC 123177 . PMID 12089327.
- ↑ Aguilar-Bryan L, Clement JP, Gonzalez G, Kunjilwar K, Babenko A, Bryan J (January 1998). "Toward understanding the assembly and structure of KATP channels". Physiol. Rev. 78 (1): 227–45. doi:10.1152/physrev.1998.78.1.227. PMID 9457174. S2CID 11851627.
- ↑ Moritz W, Leech CA, Ferrer J, Habener JF (January 2001). "Regulated expression of adenosine triphosphate-sensitive potassium channel subunits in pancreatic beta-cells". Endocrinology. 142 (1): 129–38. doi: 10.1210/en.142.1.129 . PMID 11145575.
- ↑ Akao M, Ohler A, O'Rourke B, Marbán E (June 2001). "Mitochondrial ATP-sensitive potassium channels inhibit apoptosis induced by oxidative stress in cardiac cells". Circ. Res. 88 (12): 1267–75. doi: 10.1161/hh1201.092094 . PMID 11420303.
- ↑ Crawford RM, Jovanović S, Budas GR, Davies AM, Lad H, Wenger RH, Robertson KA, Roy DJ, Ranki HJ, Jovanović A (August 2003). "Chronic mild hypoxia protects heart-derived H9c2 cells against acute hypoxia/reoxygenation by regulating expression of the SUR2A subunit of the ATP-sensitive K+ channel". J. Biol. Chem. 278 (33): 31444–55. doi: 10.1074/jbc.M303051200 . PMC 2134977 . PMID 12791696.
- ↑ Ren Y, Xu X, Wang X (December 2003). "Altered mRNA expression of ATP-sensitive and inward rectifier potassium channel subunits in streptozotocin-induced diabetic rat heart and aorta". J. Pharmacol. Sci. 93 (4): 478–83. doi: 10.1254/jphs.93.478 . PMID 14737020.
- 1 2 Craig TJ, Ashcroft FM, Prokes P (July 2008). "How ATP Inhibits the open KATP Channel". J. Gen. Physiol. 132 (1): 131–144. doi:10.1085/jgp.200709874. PMC 2442177 . PMID 18591420.
- ↑ Markworth E, Schwanstecher C, Schwanstecher M (September 2000). "ATP4- mediates closure of pancreatic beta-cell ATP-sensitive potassium channels by interaction with 1 of 4 identical sites". Diabetes. 49 (9): 1413–8. doi:10.2337/diabetes.49.9.1413. PMID 10969823.
- ↑ Koster J, Marshall B, Ensor N, Corbett J, Nichols C (2000). "Targeted Overactivity of β Cell KATP Channels Induces Profound Neonatal Diabetes". Cell. 100 (6): 645–654. doi: 10.1016/S0092-8674(00)80701-1 . PMID 10761930. S2CID 16641599.
- ↑ Koster JC, Knopp A, Flagg TP, Markova KP, Sha Q, Enkvetchakul D, Betsuyaku T, Yamada KA, Nichols CG (2001). "Tolerance for ATP-Insensitive KATP Channels in Transgenic Mice". Circulation Research. 89 (11): 1022–9. doi: 10.1161/hh2301.100342 . PMID 11717159.
- ↑ Xu M, Wang Y, Ayub A, Ashraf M (September 2001). "Mitochondrial K(ATP) channel activation reduces anoxic injury by restoring mitochondrial membrane potential". Am. J. Physiol. Heart Circ. Physiol. 281 (3): H1295–303. doi:10.1152/ajpheart.2001.281.3.H1295. PMID 11514300. S2CID 19081999.
- ↑ Mubagwa K, Flameng W (October 2001). "Adenosine, adenosine receptors and myocardial protection: an updated overview". Cardiovasc. Res. 52 (1): 25–39. doi: 10.1016/S0008-6363(01)00358-3 . PMID 11557231. S2CID 7442045.
- ↑ Suzuki M, Saito T, Sato T, Tamagawa M, Miki T, Seino S, Nakaya H (February 2003). "Cardioprotective effect of diazoxide is mediated by activation of sarcolemmal but not mitochondrial ATP-sensitive potassium channels in mice". Circulation. 107 (5): 682–5. doi:10.1161/01.CIR.0000055187.67365.81. PMID 12578868. S2CID 56186961.
- ↑ Gong B, Miki T, Seino S, Renaud JM (November 2000). "A K(ATP) channel deficiency affects resting tension, not contractile force, during fatigue in skeletal muscle". Am. J. Physiol., Cell Physiol. 279 (5): C1351–8. doi:10.1152/ajpcell.2000.279.5.C1351. PMID 11029282. S2CID 25717677.
- ↑ Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A (October 2002). "Kir6.2 is required for adaptation to stress". Proc. Natl. Acad. Sci. U.S.A. 99 (20): 13278–83. Bibcode:2002PNAS...9913278Z. doi: 10.1073/pnas.212315199 . PMC 130624 . PMID 12271142.
- ↑ Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM (July 2002). "Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels". J. Clin. Invest. 110 (2): 203–8. doi:10.1172/JCI15672. PMC 151064 . PMID 12122112.
- ↑ Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O'Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang YP, Alekseev AE, Terzic A (April 2004). "ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating". Nat. Genet. 36 (4): 382–7. doi:10.1038/ng1329. PMC 1995438 . PMID 15034580.