Transient receptor potential channels are a group of ion channels located mostly on the plasma membrane of numerous animal cell types. Most of these are grouped into two broad groups: Group 1 includes TRPC, TRPV, TRPVL, TRPM, TRPS, TRPN, and TRPA. Group 2 consists of TRPP and TRPML. Other less-well categorized TRP channels exist, including yeast channels and a number of Group 1 and Group 2 channels present in non-animals. Many of these channels mediate a variety of sensations such as pain, temperature, different kinds of tastes, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and used in animals to sense hot or cold. Some TRP channels are activated by molecules found in spices like garlic (allicin), chili pepper (capsaicin), wasabi ; others are activated by menthol, camphor, peppermint, and cooling agents; yet others are activated by molecules found in cannabis or stevia. Some act as sensors of osmotic pressure, volume, stretch, and vibration. Most of the channels are activated or inhibited by signaling lipids and contribute to a family of lipid-gated ion channels.

The transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. Fatty acid metabolites with affinity for this receptor are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA). The function of TRPV1 is detection and regulation of body temperature. In addition, TRPV1 provides a sensation of scalding heat and pain (nociception). In primary afferent sensory neurons, it cooperates with TRPA1 to mediate the detection of noxious environmental stimuli.
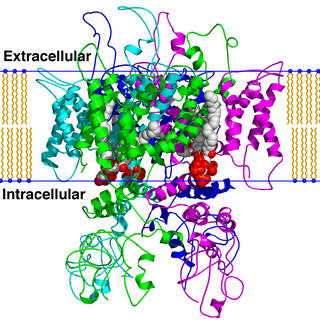
TRPV is a family of transient receptor potential cation channels in animals. All TRPVs are highly calcium selective.

TRPA is a family of transient receptor potential ion channels. The TRPA family is made up of 7 subfamilies: TRPA1, TRPA- or TRPA1-like, TRPA5, painless, pyrexia, waterwitch, and HsTRPA. TRPA1 is the only subfamily widely expressed across animals, while the other subfamilies are largely absent in deuterostomes.

Transient receptor potential cation channel, subfamily A, member 1, also known as transient receptor potential ankyrin 1, TRPA1, or The Wasabi Receptor, is a protein that in humans is encoded by the TRPA1 gene.

Transient receptor potential cation channel subfamily V member 2 is a protein that in humans is encoded by the TRPV2 gene. TRPV2 is a nonspecific cation channel that is a part of the TRP channel family. This channel allows the cell to communicate with its extracellular environment through the transfer of ions, and responds to noxious temperatures greater than 52 °C. It has a structure similar to that of potassium channels, and has similar functions throughout multiple species; recent research has also shown multiple interactions in the human body.

Transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8), also known as the cold and menthol receptor 1 (CMR1), is a protein that in humans is encoded by the TRPM8 gene. The TRPM8 channel is the primary molecular transducer of cold somatosensation in humans. In addition, mints can desensitize a region through the activation of TRPM8 receptors.

Transient receptor potential cation channel subfamily M member 3 is a protein that in humans is encoded by the TRPM3 gene.

Transient receptor potential cation channel, subfamily V, member 3, also known as TRPV3, is a human gene encoding the protein of the same name.

Transient receptor potential cation channel subfamily V member 5 is a calcium channel protein that in humans is encoded by the TRPV5 gene.

N-Arachidonyl glycine receptor, also known as G protein-coupled receptor 18 (GPR18), is a protein that in humans is encoded by the GPR18 gene. Along with the other previously "orphan" receptors GPR55 and GPR119, GPR18 has been found to be a receptor for endogenous lipid neurotransmitters, several of which also bind to cannabinoid receptors. It has been found to be involved in the regulation of intraocular pressure.

Iodoresiniferatoxin (I-RTX) is a strong competitive antagonist of the Transient Receptor Potential Vanilloid 1 (TRPV1) receptor. I-RTX is derived from resiniferatoxin (RTX).
Relief from chronic pain remains a recognized unmet medical need. Consequently, the search for new analgesic agents is being intensively studied by the pharmaceutical industry. The TRPV1 receptor is a ligand gated ion channel that has been implicated in mediation of many types of pain and therefore studied most extensively. The first competitive antagonist, capsazepine, was first described in 1990; since then, several TRPV1 antagonists have entered clinical trials as analgesic agents. Should these new chemical entities relieve symptoms of chronic pain, then this class of compounds may offer one of the first novel mechanisms for the treatment of pain in many years.
Zucapsaicin (Civanex) is a medication used to treat osteoarthritis of the knee and other neuropathic pain. It is applied three times daily for a maximum of three months. Zucapsaicin is a member of phenols and a member of methoxybenzenes. It is a modulator of transient receptor potential cation channel subfamily V member 1 (TRPV-1), also known as the vanilloid or capsaicin receptor 1 that reduces pain, and improves articular functions. It is the cis-isomer of capsaicin. Civamide, manufactured by Winston Pharmaceuticals, is produced in formulations for oral, nasal, and topical use.
Specialized pro-resolving mediators are a large and growing class of cell signaling molecules formed in cells by the metabolism of polyunsaturated fatty acids (PUFA) by one or a combination of lipoxygenase, cyclooxygenase, and cytochrome P450 monooxygenase enzymes. Pre-clinical studies, primarily in animal models and human tissues, implicate SPM in orchestrating the resolution of inflammation. Prominent members include the resolvins and protectins.

GSK1016790A is a drug developed by GlaxoSmithKline which acts as a potent and selective agonist for the TRPV4 receptor. It has been used to study the role of TRPV4 receptors in the function of smooth muscle tissue, particularly that lining blood vessels, lymphatic system, and the bladder.

A-967079 is a drug which acts as a potent and selective antagonist for the TRPA1 receptor. It has analgesic and antiinflammatory effects and is used in scientific research, but has not been developed for medical use.

AMG-9810 is a drug which acts as a potent and selective antagonist for the TRPV1 receptor. It has analgesic and antiinflammatory effects and is used in scientific research, but has not been developed for medical use. It has high antagonist potency and good bioavailability and pharmacokinetics, and so has been used to study the role of TRPV1 in areas other than pain perception, such as its roles in the brain.

HC-067047 is a drug which acts as a potent and selective antagonist for the TRPV4 receptor. It has been used to investigate the role of TRPV4 receptors in a number of areas, such as regulation of blood pressure, bladder function and some forms of pain, as well as neurological functions.

AMG-517 is a drug which acts as a potent and selective blocker of the TRPV1 ion channel. It was developed as a potential treatment for chronic pain, but while it was an effective analgesic in animal studies it was dropped from human clinical trials at Phase I due to producing hyperthermia as a side effect, as well as poor water solubility. It is still used in scientific research into the function of the TRPV1 channel and its role in pain and inflammation, and has been used as a template for the design of several newer analogues which have improved properties.




















