
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.

An acetylcholine receptor is an integral membrane protein that responds to the binding of acetylcholine, a neurotransmitter.
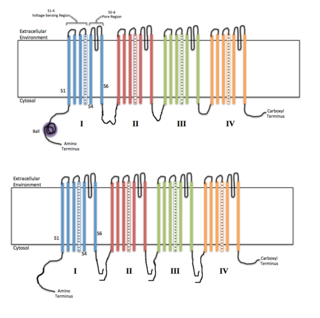
Voltage-gated ion channels are a class of transmembrane proteins that form ion channels that are activated by changes in the electrical membrane potential near the channel. The membrane potential alters the conformation of the channel proteins, regulating their opening and closing. Cell membranes are generally impermeable to ions, thus they must diffuse through the membrane through transmembrane protein channels. They have a crucial role in excitable cells such as neuronal and muscle tissues, allowing a rapid and co-ordinated depolarization in response to triggering voltage change. Found along the axon and at the synapse, voltage-gated ion channels directionally propagate electrical signals. Voltage-gated ion-channels are usually ion-specific, and channels specific to sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) ions have been identified. The opening and closing of the channels are triggered by changing ion concentration, and hence charge gradient, between the sides of the cell membrane.

Cyclic nucleotide–gated ion channels or CNG channels are ion channels that function in response to the binding of cyclic nucleotides. CNG channels are nonselective cation channels that are found in the membranes of various tissue and cell types, and are significant in sensory transduction as well as cellular development. Their function can be the result of a combination of the binding of cyclic nucleotides and either a depolarization or a hyperpolarization event. Initially discovered in the cells that make up the retina of the eye, CNG channels have been found in many different cell types across both the animal and the plant kingdoms. CNG channels have a very complex structure with various subunits and domains that play a critical role in their function. CNG channels are significant in the function of various sensory pathways including vision and olfaction, as well as in other key cellular functions such as hormone release and chemotaxis. CNG channels have also been found to exist in prokaryotes, including many spirochaeta, though their precise role in bacterial physiology remains unknown.

Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in response to the binding of a chemical messenger (i.e. a ligand), such as a neurotransmitter.

Chloride channels are a superfamily of poorly understood ion channels specific for chloride. These channels may conduct many different ions, but are named for chloride because its concentration in vivo is much higher than other anions. Several families of voltage-gated channels and ligand-gated channels have been characterized in humans.
Channelrhodopsins are a subfamily of retinylidene proteins (rhodopsins) that function as light-gated ion channels. They serve as sensory photoreceptors in unicellular green algae, controlling phototaxis: movement in response to light. Expressed in cells of other organisms, they enable light to control electrical excitability, intracellular acidity, calcium influx, and other cellular processes. Channelrhodopsin-1 (ChR1) and Channelrhodopsin-2 (ChR2) from the model organism Chlamydomonas reinhardtii are the first discovered channelrhodopsins. Variants that are sensitive to different colors of light or selective for specific ions have been cloned from other species of algae and protists.

Photostimulation is the use of light to artificially activate biological compounds, cells, tissues, or even whole organisms. Photostimulation can be used to noninvasively probe various relationships between different biological processes, using only light. In the long run, photostimulation has the potential for use in different types of therapy, such as migraine headache. Additionally, photostimulation may be used for the mapping of neuronal connections between different areas of the brain by “uncaging” signaling biomolecules with light. Therapy with photostimulation has been called light therapy, phototherapy, or photobiomodulation.

Guard cells are specialized plant cells in the epidermis of leaves, stems and other organs that are used to control gas exchange. They are produced in pairs with a gap between them that forms a stomatal pore. The stomatal pores are largest when water is freely available and the guard cells become turgid, and closed when water availability is critically low and the guard cells become flaccid. Photosynthesis depends on the diffusion of carbon dioxide (CO2) from the air through the stomata into the mesophyll tissues. Oxygen (O2), produced as a byproduct of photosynthesis, exits the plant via the stomata. When the stomata are open, water is lost by evaporation and must be replaced via the transpiration stream, with water taken up by the roots. Plants must balance the amount of CO2 absorbed from the air with the water loss through the stomatal pores, and this is achieved by both active and passive control of guard cell turgor pressure and stomatal pore size.
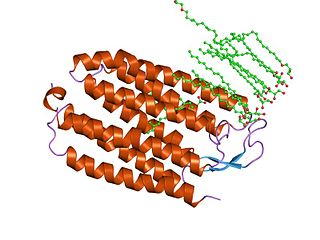
Halorhodopsin is a seven-transmembrane retinylidene protein from microbial rhodopsin family. It is a chloride-specific light-gated ion pump found in archaea known as halobacteria. It is activated by green light wavelengths of approximately 578nm. Halorhodopsin also shares sequence similarity to channelrhodopsin, another light-driven ion channel.
Retinylidene proteins, or rhodopsins in a broad sense, are proteins that use retinal as a chromophore for light reception. They are the molecular basis for a variety of light-sensing systems from phototaxis in flagellates to eyesight in animals. Retinylidene proteins include all forms of opsin and rhodopsin. While rhodopsin in the narrow sense refers to a dim-light visual pigment found in vertebrates, usually on rod cells, rhodopsin in the broad sense refers to any molecule consisting of an opsin and a retinal chromophore in the ground state. When activated by light, the chromophore is isomerized, at which point the molecule as a whole is no longer rhodopsin, but a related molecule such as metarhodopsin. However, it remains a retinylidene protein. The chromophore then separates from the opsin, at which point the bare opsin is a retinylidene protein. Thus, the molecule remains a retinylidene protein throughout the phototransduction cycle.
Optogenetics is a biological technique to control the activity of neurons or other cell types with light. This is achieved by expression of light-sensitive ion channels, pumps or enzymes specifically in the target cells. On the level of individual cells, light-activated enzymes and transcription factors allow precise control of biochemical signaling pathways. In systems neuroscience, the ability to control the activity of a genetically defined set of neurons has been used to understand their contribution to decision making, learning, fear memory, mating, addiction, feeding, and locomotion. In a first medical application of optogenetic technology, vision was partially restored in a blind patient.

Cell surface receptors are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving extracellular molecules. They are specialized integral membrane proteins that allow communication between the cell and the extracellular space. The extracellular molecules may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules, or nutrients; they react with the receptor to induce changes in the metabolism and activity of a cell. In the process of signal transduction, ligand binding affects a cascading chemical change through the cell membrane.
Mechanosensitive channels (MSCs), mechanosensitive ion channels or stretch-gated ion channels are membrane proteins capable of responding to mechanical stress over a wide dynamic range of external mechanical stimuli. They are present in the membranes of organisms from the three domains of life: bacteria, archaea, and eukarya. They are the sensors for a number of systems including the senses of touch, hearing and balance, as well as participating in cardiovascular regulation and osmotic homeostasis (e.g. thirst). The channels vary in selectivity for the permeating ions from nonselective between anions and cations in bacteria, to cation selective allowing passage Ca2+, K+ and Na+ in eukaryotes, and highly selective K+ channels in bacteria and eukaryotes.
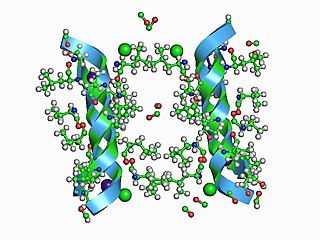
In electrophysiology, the term gating refers to the opening (activation) or closing of ion channels. This change in conformation is a response to changes in transmembrane voltage.

Karl Alexander Deisseroth is an American scientist. He is the D.H. Chen Professor of Bioengineering and of psychiatry and behavioral sciences at Stanford University.

Peter Hegemann is a Hertie Senior Research Chair for Neurosciences and a Professor of Experimental Biophysics at the Department of Biology, Faculty of Life Sciences, Humboldt University of Berlin, Germany. He is known for his discovery of channelrhodopsin, a type of ion channels regulated by light, thereby serving as a light sensor. This created the field of optogenetics, a technique that controls the activities of specific neurons by applying light. He has received numerous accolades, including the Rumford Prize, the Shaw Prize in Life Science and Medicine, and the Albert Lasker Award for Basic Medical Research.
Microbial rhodopsins, also known as bacterial rhodopsins, are retinal-binding proteins that provide light-dependent ion transport and sensory functions in halophilic and other bacteria. They are integral membrane proteins with seven transmembrane helices, the last of which contains the attachment point for retinal.

Anion-conducting channelrhodopsins are light-gated ion channels that open in response to light and let negatively charged ions enter a cell. All channelrhodopsins use retinal as light-sensitive pigment, but they differ in their ion selectivity. Anion-conducting channelrhodopsins are used as tools to manipulate brain activity in mice, fruit flies and other model organisms (Optogenetics). Neurons expressing anion-conducting channelrhodopsins are silenced when illuminated with light, an effect that has been used to investigate information processing in the brain. For example, suppressing dendritic calcium spikes in specific neurons with light reduced the ability of mice to perceive a light touch to a whisker. Studying how the behavior of an animal changes when specific neurons are silenced allows scientists to determine the role of these neurons in the complex circuits controlling behavior.

Georg Nagel is a biophysicist and professor at the Department for Neurophysiology at the University of Würzburg in Germany. His research is focused on microbial photoreceptors and the development of optogenetic tools.
















