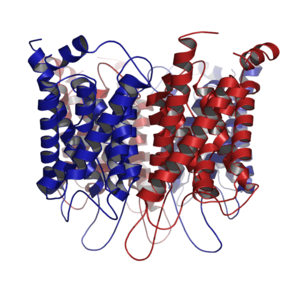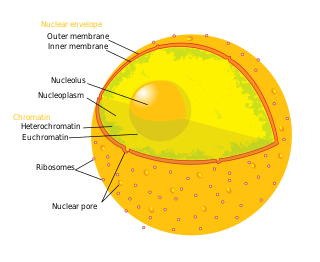
A nuclear pore is a channel as part of the nuclear pore complex (NPC), a large protein complex found in the nuclear envelope of eukaryotic cells. The nuclear envelope surrounds the cell nucleus containing DNA and facilitates the selective membrane transport of various molecules.

Diabetes insipidus (DI), alternately called arginine vasopressin deficiency (AVP-D) or arginine vasopressin resistance (AVP-R), is a condition characterized by large amounts of dilute urine and increased thirst. The amount of urine produced can be nearly 20 liters per day. Reduction of fluid has little effect on the concentration of the urine. Complications may include dehydration or seizures.

Semipermeable membrane is a type of biological or synthetic, polymeric membrane that allows certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of an egg.
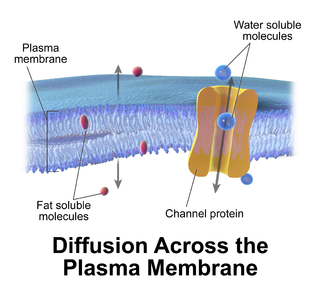
Passive transport is a type of membrane transport that does not require energy to move substances across cell membranes. Instead of using cellular energy, like active transport, passive transport relies on the second law of thermodynamics to drive the movement of substances across cell membranes. Fundamentally, substances follow Fick's first law, and move from an area of high concentration to an area of low concentration because this movement increases the entropy of the overall system. The rate of passive transport depends on the permeability of the cell membrane, which, in turn, depends on the organization and characteristics of the membrane lipids and proteins. The four main kinds of passive transport are simple diffusion, facilitated diffusion, filtration, and/or osmosis.
A membrane transport protein is a membrane protein involved in the movement of ions, small molecules, and macromolecules, such as another protein, across a biological membrane. Transport proteins are integral transmembrane proteins; that is they exist permanently within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion, active transport, osmosis, or reverse diffusion. The two main types of proteins involved in such transport are broadly categorized as either channels or carriers. Examples of channel/carrier proteins include the GLUT 1 uniporter, sodium channels, and potassium channels. The solute carriers and atypical SLCs are secondary active or facilitative transporters in humans. Collectively membrane transporters and channels are known as the transportome. Transportomes govern cellular influx and efflux of not only ions and nutrients but drugs as well.
Nephrogenic diabetes insipidus,, is a form of diabetes insipidus primarily due to pathology of the kidney. This is in contrast to central or neurogenic diabetes insipidus, which is caused by insufficient levels of vasopressin. Nephrogenic diabetes insipidus is caused by an improper response of the kidney to vasopressin, leading to a decrease in the ability of the kidney to concentrate the urine by removing free water.

Vasopressin receptor 2 (V2R), or arginine vasopressin receptor 2, is a protein that acts as receptor for vasopressin. AVPR2 belongs to the subfamily of G-protein-coupled receptors. Its activity is mediated by the Gs type of G proteins, which stimulate adenylate cyclase.
The actions of vasopressin are mediated by stimulation of tissue-specific G protein-coupled receptors (GPCRs) called vasopressin receptors that are classified into the V1 (V1A), V2, and V3 (V1B) receptor subtypes. These three subtypes differ in localization, function and signal transduction mechanisms.
Aquaporin-4, also known as AQP-4, is a water channel protein encoded by the AQP4 gene in humans. AQP-4 belongs to the aquaporin family of integral membrane proteins that conduct water through the cell membrane. A limited number of aquaporins are found within the central nervous system (CNS): AQP1, 3, 4, 5, 8, 9, and 11, but more exclusive representation of AQP1, 4, and 9 are found in the brain and spinal cord. AQP4 shows the largest presence in the cerebellum and spinal cord grey matter. In the CNS, AQP4 is the most prevalent aquaporin channel, specifically located at the perimicrovessel astrocyte foot processes, glia limitans, and ependyma. In addition, this channel is commonly found facilitating water movement near cerebrospinal fluid and vasculature.

Aquaporin-2 (AQP-2) is found in the apical cell membranes of the kidney's collecting duct principal cells and in intracellular vesicles located throughout the cell. It is encoded by the AQP2 gene.

Aquaporin 3 (AQP-3) is the protein product of the human AQP3 gene. It is found in the basolateral cell membrane of principal collecting duct cells and provides a pathway for water to exit these cells. Aquaporin-3 is also permeable to glycerol, ammonia, urea, and hydrogen peroxide. It is expressed in various tissues including the skin, respiratory tract, and kidneys as well as various types of cancers. In the kidney, aquaproin-3 is unresponsive to the antidiuretic hormone vasopressin, unlike aquaporin-2. This protein is also a determinant for the GIL blood group system.

Aquaporin 1 (AQP-1) is a protein that in humans is encoded by the AQP1 gene.
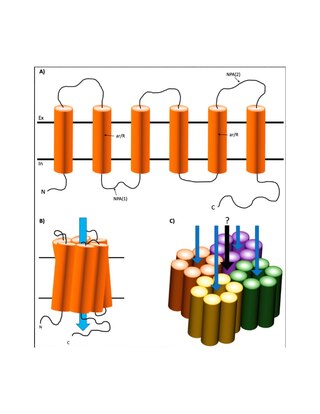
Major intrinsic proteins comprise a large superfamily of transmembrane protein channels that are grouped together on the basis of homology. The MIP superfamily includes three subfamilies: aquaporins, aquaglyceroporins and S-aquaporins.
- The aquaporins (AQPs) are water selective.
- The aquaglyceroporins are permeable to water, but also to other small uncharged molecules such as glycerol.
- The third subfamily, with little conserved amino acid sequences around the NPA boxes, include 'superaquaporins' (S-aquaporins).
A urea transporter is a membrane transport protein, transporting urea. Humans and other mammals have two types of urea transport proteins, UT-A and UT-B. The UT-A proteins are important for renal urea handling and are produced by alternative splicing of the SLC14A2 gene. Urea transport in the kidney is regulated by vasopressin.

Lens fiber major intrinsic protein also known as aquaporin-0 is a protein that in humans is encoded by the MIP gene.

Aquaporin-5 (AQP-5) is a protein that in humans is encoded by the AQP5 gene.

Aquaporin-9 (AQP-9) is a protein that in humans is encoded by the AQP9 gene.

Aquaporin-7 (AQP-7) is a protein that in humans is encoded by the AQP7 gene.
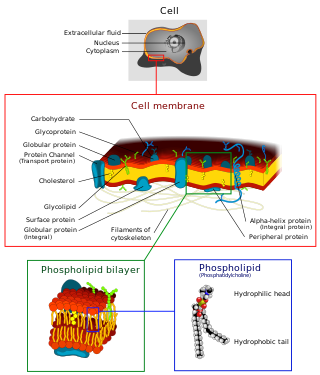
The cell membrane is a biological membrane that separates and protects the interior of a cell from the outside environment. The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of a cell, being selectively permeable to ions and organic molecules. In addition, cell membranes are involved in a variety of cellular processes such as cell adhesion, ion conductivity, and cell signalling and serve as the attachment surface for several extracellular structures, including the cell wall and the carbohydrate layer called the glycocalyx, as well as the intracellular network of protein fibers called the cytoskeleton. In the field of synthetic biology, cell membranes can be artificially reassembled.

Aquaporin-6, (AQP-6) also known as kidney-specific aquaporin is a protein in humans that is encoded by the AQP6 gene.