
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.

Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms' cells. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction of all muscle cell types, and in fertilization. Many enzymes require calcium ions as a cofactor, including several of the coagulation factors. Extracellular calcium is also important for maintaining the potential difference across excitable cell membranes, as well as proper bone formation.

Calcium metabolism is the movement and regulation of calcium ions (Ca2+) in (via the gut) and out (via the gut and kidneys) of the body, and between body compartments: the blood plasma, the extracellular and intracellular fluids, and bone. Bone acts as a calcium storage center for deposits and withdrawals as needed by the blood via continual bone remodeling.
Transient receptor potential channels are a group of ion channels located mostly on the plasma membrane of numerous animal cell types. Most of these are grouped into two broad groups: Group 1 includes TRPC, TRPV, TRPVL, TRPM, TRPS, TRPN TRPA. Group 2 consists of TRPP and TRPML. Other less-well categorized TRP channels exist, including yeast channels and a number of Group 1 and Group 2 channels present in non-animals. Many of these channels mediate a variety of sensations such as pain, temperature, different kinds of tastes, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and used in animals to sense hot or cold. Some TRP channels are activated by molecules found in spices like garlic (allicin), chili pepper (capsaicin), wasabi ; others are activated by menthol, camphor, peppermint, and cooling agents; yet others are activated by molecules found in cannabis or stevia. Some act as sensors of osmotic pressure, volume, stretch, and vibration. Most of the channels are activated or inhibited by signaling lipids and contribute to a family of lipid-gated ion channels.
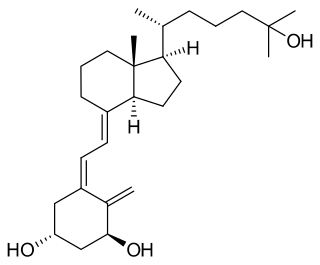
Calcitriol is the active form of vitamin D, normally made in the kidney. It is also known as 1,25-dihydroxycholecalciferol. It is a hormone which binds to and activates the vitamin D receptor in the nucleus of the cell, which then increases the expression of many genes. Calcitriol increases blood calcium (Ca2+) mainly by increasing the uptake of calcium from the intestines.

Klotho is an enzyme that in humans is encoded by the KL gene. The three subfamilies of klotho are α-klotho, β-klotho, and γ-klotho. α-klotho activates FGF23, and β-klotho activates FGF19 and FGF21. When the subfamily is not specified, the word "klotho" typically refers to the α-klotho subfamily, because α-klotho was discovered before the other members.

Calbindins are three different calcium-binding proteins: calbindin, calretinin and S100G. They were originally described as vitamin D-dependent calcium-binding proteins in the intestine and kidney of chicks and mammals. They are now classified in different subfamilies as they differ in the number of Ca2+ binding EF hands.

The transient receptor potential cation channel subfamily V member 1 (TRPV1), also known as the capsaicin receptor and the vanilloid receptor 1, is a protein that, in humans, is encoded by the TRPV1 gene. It was the first isolated member of the transient receptor potential vanilloid receptor proteins that in turn are a sub-family of the transient receptor potential protein group. This protein is a member of the TRPV group of transient receptor potential family of ion channels. Fatty acid metabolites with affinity for this receptor are produced by cyanobacteria, which diverged from eukaryotes at least 2000 million years ago (MYA). The function of TRPV1 is detection and regulation of body temperature. In addition, TRPV1 provides a sensation of scalding heat and pain (nociception). In primary afferent sensory neurons, it cooperates with TRPA1 to mediate the detection of noxious environmental stimuli.
TRPC is a family of transient receptor potential cation channels in animals.
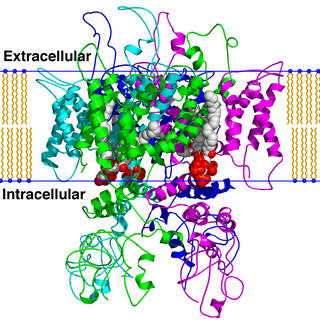
TRPV is a family of transient receptor potential cation channels in animals. All TRPVs are highly calcium selective.

Transient receptor potential canonical 1 (TRPC1) is a protein that in humans is encoded by the TRPC1 gene.

Transient receptor potential cation channel, subfamily M, member 2, also known as TRPM2, is a protein that in humans is encoded by the TRPM2 gene.

Transient receptor potential cation channel subfamily M member 5 (TRPM5), also known as long transient receptor potential channel 5 is a protein that in humans is encoded by the TRPM5 gene.

Transient receptor potential cation channel subfamily V member 2 is a protein that in humans is encoded by the TRPV2 gene. TRPV2 is a nonspecific cation channel that is a part of the TRP channel family. This channel allows the cell to communicate with its extracellular environment through the transfer of ions, and responds to noxious temperatures greater than 52 °C. It has a structure similar to that of potassium channels, and has similar functions throughout multiple species; recent research has also shown multiple interactions in the human body.

Transient receptor potential cation channel subfamily M member 4 (hTRPM4), also known as melastatin-4, is a protein that in humans is encoded by the TRPM4 gene.

Transient receptor potential cation channel subfamily M (melastatin) member 8 (TRPM8), also known as the cold and menthol receptor 1 (CMR1), is a protein that in humans is encoded by the TRPM8 gene. The TRPM8 channel is the primary molecular transducer of cold somatosensation in humans. In addition, mints can desensitize a region through the activation of TRPM8 receptors.

Transient receptor potential cation channel subfamily M member 3 is a protein that in humans is encoded by the TRPM3 gene.

Transient receptor potential cation channel subfamily V member 5 is a calcium channel protein that in humans is encoded by the TRPV5 gene.

Leukotriene B4 receptor 2, also known as BLT2, BLT2 receptor, and BLTR2, is an Integral membrane protein that is encoded by the LTB4R2 gene in humans and the Ltbr2 gene in mice.
The transient receptor potential Ca2+ channel (TRP-CC) family (TC# 1.A.4) is a member of the voltage-gated ion channel (VIC) superfamily and consists of cation channels conserved from worms to humans. The TRP-CC family also consists of seven subfamilies (TRPC, TRPV, TRPM, TRPN, TRPA, TRPP, and TRPML) based on their amino acid sequence homology:
- the canonical or classic TRPs,
- the vanilloid receptor TRPs,
- the melastatin or long TRPs,
- ankyrin (whose only member is the transmembrane protein 1 [TRPA1])
- TRPN after the nonmechanoreceptor potential C (nonpC), and the more distant cousins,
- the polycystins
- and mucolipins.





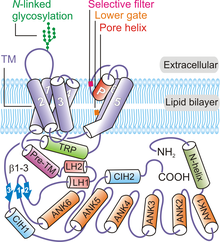


![Figure 4. Role of TRPV6 in intestinal calcium absorption. TRPV6 mediates Ca entry across the plasma membrane as the first step in the transcellular pathway of Ca transport. This is considered the rate-limiting step in Ca absorption by the enterocytes. The transcellular pathway enables the transport of Ca against a [Ca ] gradient to ensure Ca absorption when the luminal [Ca ] is lower than that in the blood side; The Ca binding protein calbindin-D9k and plasma membrane Ca ATPase (PMCA) are known components in these transcellular pathways. Figure 4 TRPV6 transcellular pathway.png](http://upload.wikimedia.org/wikipedia/commons/thumb/2/23/Figure_4_TRPV6_transcellular_pathway.png/220px-Figure_4_TRPV6_transcellular_pathway.png)
















