
The placenta is a temporary embryonic and later fetal organ that begins developing from the blastocyst shortly after implantation. It plays critical roles in facilitating nutrient, gas and waste exchange between the physically separate maternal and fetal circulations, and is an important endocrine organ, producing hormones that regulate both maternal and fetal physiology during pregnancy. The placenta connects to the fetus via the umbilical cord, and on the opposite aspect to the maternal uterus in a species-dependent manner. In humans, a thin layer of maternal decidual (endometrial) tissue comes away with the placenta when it is expelled from the uterus following birth. Placentas are a defining characteristic of placental mammals, but are also found in marsupials and some non-mammals with varying levels of development.

The amnion is a membrane that closely covers human and various other embryos when they first form. It fills with amniotic fluid, which causes the amnion to expand and become the amniotic sac that provides a protective environment for the developing embryo. The amnion, along with the chorion, the yolk sac and the allantois protect the embryo. In birds, reptiles and monotremes, the protective sac is enclosed in a shell. In marsupials and placental mammals, it is enclosed in a uterus.
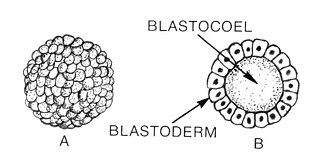
Blastulation is the stage in early animal embryonic development that produces the blastula. In mammalian development the blastula develops into the blastocyst with a differentiated inner cell mass and an outer trophectoderm. The blastula is a hollow sphere of cells known as blastomeres surrounding an inner fluid-filled cavity called the blastocoel. Embryonic development begins with a sperm fertilizing an egg cell to become a zygote, which undergoes many cleavages to develop into a ball of cells called a morula. Only when the blastocoel is formed does the early embryo become a blastula. The blastula precedes the formation of the gastrula in which the germ layers of the embryo form.

The chorion is the outermost fetal membrane around the embryo in mammals, birds and reptiles (amniotes). It develops from an outer fold on the surface of the yolk sac, which lies outside the zona pellucida, known as the vitelline membrane in other animals. In insects, it is developed by the follicle cells while the egg is in the ovary. Some mollusks also have chorions as part of their eggs. For example, fragile octopus eggs have only a chorion as their envelope.

The blastocyst is a structure formed in the early embryonic development of mammals. It possesses an inner cell mass (ICM) also known as the embryoblast which subsequently forms the embryo, and an outer layer of trophoblast cells called the trophectoderm. This layer surrounds the inner cell mass and a fluid-filled cavity known as the blastocoel. In the late blastocyst, the trophectoderm is known as the trophoblast. The trophoblast gives rise to the chorion and amnion, the two fetal membranes that surround the embryo. The placenta derives from the embryonic chorion and the underlying uterine tissue of the mother.

A molar pregnancy, also known as a hydatidiform mole, is an abnormal form of pregnancy in which a non-viable fertilized egg implants in the uterus. It falls under the category of gestational trophoblastic diseases. During a molar pregnancy, the uterus contains a growing mass characterized by swollen chorionic villi, resembling clusters of grapes. The occurrence of a molar pregnancy can be attributed to the fertilized egg lacking an original maternal nucleus. As a result, the products of conception may or may not contain fetal tissue. These molar pregnancies are categorized into two types: partial moles and complete moles, where the term 'mole' simply denotes a clump of growing tissue or a ‘growth
Gestational choriocarcinoma is a form of gestational trophoblastic neoplasia, which is a type of gestational trophoblastic disease (GTD), that can occur during pregnancy. It is a rare disease where the trophoblast, a layer of cells surrounding the blastocyst, undergoes abnormal developments, leading to trophoblastic tumors. The choriocarcinoma can metastasize to other organs, including the lungs, kidney, and liver. The amount and degree of choriocarcinoma spread to other parts of the body can vary greatly from person to person.
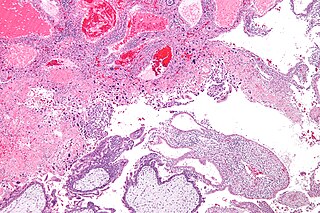
Gestational trophoblastic neoplasia (GTN) is group of rare diseases related to pregnancy and included in gestational trophoblastic disease (GTD) in which abnormal trophoblast cells grow in the uterus. GTN can be classified into benign and malignant lesions. Benign lesions include placental site nodule and hydatidiform moles while malignant lesions have four subtypes including invasive mole, gestational choriocarcinoma, placental site trophoblastic tumor (PSTT) and epithelioid trophoblastic tumor (ETT). The choriocarcinoma has 2 significant subtypes including gestational and non-gestational and they are differentiated by their different biological feature and prognosis. Signs and symptoms of GTN will appear vary from person to person and depending upon the type of the disease. They may include uterine bleeding not related to menstruation, pain or pressure in pelvis, large uterus and high blood pressure during pregnancy. The cause of this disease is unknown but the identification of the tumor based on total beta-human chorionic gonadotropin (β-hCG) in the serum.

The decidua is the modified mucosal lining of the uterus that forms every month, in preparation for pregnancy. It is shed off each month when there is no fertilised egg to support. The decidua is under the influence of progesterone. Endometrial cells become highly characteristic. The decidua forms the maternal part of the placenta and remains for the duration of the pregnancy. After birth the decidua is shed together with the placenta.

The syncytiotrophoblast is the epithelial covering of the highly vascular embryonic placental villi, which invades the wall of the uterus to establish nutrient circulation between the embryo and the mother. It is a multinucleate, terminally differentiated syncytium, extending to 13 cm.

"Cytotrophoblast" is the name given to both the inner layer of the trophoblast or the cells that live there. It is interior to the syncytiotrophoblast and external to the wall of the blastocyst in a developing embryo.
Hofbauer cells are oval eosinophilic histiocytes with granules and vacuoles found in the placenta, which are of mesenchymal origin, in mesoderm of the chorionic villus, particularly numerous in early pregnancy.

Implantation, also known as nidation, is the stage in the mammalian embryonic development in which the blastocyst hatches, attaches, adheres, and invades into the endometrium of the female's uterus. Implantation is the first stage of gestation, and, when successful, the female is considered to be pregnant. An implanted embryo is detected by the presence of increased levels of human chorionic gonadotropin (hCG) in a pregnancy test. The implanted embryo will receive oxygen and nutrients in order to grow.

Decidualization is a process that results in significant changes to cells of the endometrium in preparation for, and during, pregnancy. This includes morphological and functional changes to endometrial stromal cells (ESCs), the presence of decidual white blood cells (leukocytes), and vascular changes to maternal arteries. The sum of these changes results in the endometrium changing into a structure called the decidua. In humans, the decidua is shed during childbirth.

Human embryonic development or human embryogenesis is the development and formation of the human embryo. It is characterised by the processes of cell division and cellular differentiation of the embryo that occurs during the early stages of development. In biological terms, the development of the human body entails growth from a one-celled zygote to an adult human being. Fertilization occurs when the sperm cell successfully enters and fuses with an egg cell (ovum). The genetic material of the sperm and egg then combine to form the single cell zygote and the germinal stage of development commences. Embryonic development in the human, covers the first eight weeks of development; at the beginning of the ninth week the embryo is termed a fetus. The eight weeks have 23 stages.

Syncytin-1 also known as enverin is a protein found in humans and other primates that is encoded by the ERVW-1 gene. Syncytin-1 is a cell-cell fusion protein whose function is best characterized in placental development. The placenta in turn aids in embryo attachment to the uterus and establishment of a nutrient supply.
Interspecific pregnancy is the pregnancy involving an embryo or fetus belonging to another species than the carrier. Strictly, it excludes the situation where the fetus is a hybrid of the carrier and another species, thereby excluding the possibility that the carrier is the biological mother of the offspring. Strictly, interspecific pregnancy is also distinguished from endoparasitism, where parasite offspring grow inside the organism of another species, not necessarily in the womb.

Intermediate trophoblast is a distinct subtype of trophoblastic tissue that arises from the cytotrophoblast.
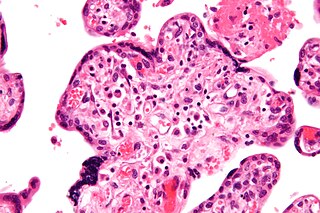
Villitis of unknown etiology (VUE), also known as chronic villitis, is a placental injury. VUE is an inflammatory condition involving the chorionic villi. VUE is a recurrent condition and can be associated with intrauterine growth restriction (IUGR). IUGR involves the poor growth of the foetus, stillbirth, miscarriage, and premature delivery. VUE recurs in about 1/3 of subsequent pregnancies.
Extravillous trophoblasts(EVTs), are one form of differentiated trophoblast cells of the placenta. They are invasive mesenchymal cells which function to establish critical tissue connection in the developing placental-uterine interface. EVTs derive from progenitor cytotrophoblasts (CYTs), as does the other main trophoblast subtype, syncytiotrophoblast (SYN). They are sometimes called intermediate trophoblast.


















