
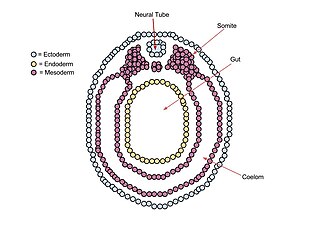
The mesoderm is the middle layer of the three germ layers that develops during gastrulation in the very early development of the embryo of most animals. The outer layer is the ectoderm, and the inner layer is the endoderm.

Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst, is reorganized into a two-layered or three-layered embryo known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.

The blastocoel, also spelled blastocoele and blastocele, and also called cleavage cavity, or segmentation cavity is a fluid-filled or yolk-filled cavity that forms in the blastula during very early embryonic development. At this stage in mammals the blastula develops into the blastocyst containing an inner cell mass, and outer trophectoderm.
A germ layer is a primary layer of cells that forms during embryonic development. The three germ layers in vertebrates are particularly pronounced; however, all eumetazoans produce two or three primary germ layers. Some animals, like cnidarians, produce two germ layers making them diploblastic. Other animals such as bilaterians produce a third layer between these two layers, making them triploblastic. Germ layers eventually give rise to all of an animal's tissues and organs through the process of organogenesis.
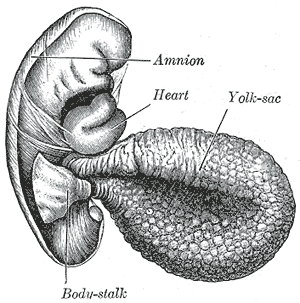
The yolk sac is a membranous sac attached to an embryo, formed by cells of the hypoblast layer of the bilaminar embryonic disc. This is alternatively called the umbilical vesicle by the Terminologia Embryologica (TE), though yolk sac is far more widely used. In humans, the yolk sac is important in early embryonic blood supply, and much of it is incorporated into the primordial gut during the fourth week of embryonic development.

In developmental biology, animal embryonic development, also known as animal embryogenesis, is the developmental stage of an animal embryo. Embryonic development starts with the fertilization of an egg cell (ovum) by a sperm cell, (spermatozoon). Once fertilized, the ovum becomes a single diploid cell known as a zygote. The zygote undergoes mitotic divisions with no significant growth and cellular differentiation, leading to development of a multicellular embryo after passing through an organizational checkpoint during mid-embryogenesis. In mammals, the term refers chiefly to the early stages of prenatal development, whereas the terms fetus and fetal development describe later stages.
A neurula is a vertebrate embryo at the early stage of development in which neurulation occurs. The neurula stage is preceded by the gastrula stage; consequentially, neurulation is preceded by gastrulation. Neurulation marks the beginning of the process of organogenesis.
The primitive node is the organizer for gastrulation in most amniote embryos. In birds it is known as Hensen's node, and in amphibians it is known as the Spemann-Mangold organizer. It is induced by the Nieuwkoop center in amphibians, or by the posterior marginal zone in amniotes including birds.

The primitive streak is a structure that forms in the early embryo in amniotes. In amphibians, the equivalent structure is the blastopore. During early embryonic development, the embryonic disc becomes oval shaped, and then pear-shaped with the broad end towards the anterior, and the narrower region projected to the posterior. The primitive streak forms a longitudinal midline structure in the narrower posterior (caudal) region of the developing embryo on its dorsal side. At first formation, the primitive streak extends for half the length of the embryo. In the human embryo, this appears by stage 6, about 17 days.

In amniote embryonic development, the epiblast is one of two distinct cell layers arising from the inner cell mass in the mammalian blastocyst, or from the blastula in reptiles and birds, the other layer is the hypoblast. It drives the embryo proper through its differentiation into the three primary germ layers, ectoderm, mesoderm and endoderm, during gastrulation. The amniotic ectoderm and extraembryonic mesoderm also originate from the epiblast.

Mesenchyme is a type of loosely organized animal embryonic connective tissue of undifferentiated cells that give rise to most tissues, such as skin, blood or bone. The interactions between mesenchyme and epithelium help to form nearly every organ in the developing embryo.

The bilaminar embryonic disc, bilaminar blastoderm or embryonic disc is the distinct two-layered structure of cells formed in an embryo. In the development of the human embryo this takes place by day eight. It is formed when the inner cell mass, also known as the embryoblast, forms a bilaminar disc of two layers, an upper layer called the epiblast and a lower layer called the hypoblast, which will eventually form into fetus. These two layers of cells are stretched between two fluid-filled cavities at either end: the primitive yolk sac and the amniotic sac.
The heart is the first functional organ in a vertebrate embryo. There are 5 stages to heart development.
In the field of developmental biology, regional differentiation is the process by which different areas are identified in the development of the early embryo. The process by which the cells become specified differs between organisms.

The development of fishes is unique in some specific aspects compared to the development of other animals.

Cerberus is a protein that in humans is encoded by the CER1 gene. Cerberus is a signaling molecule which contributes to the formation of the head, heart and left-right asymmetry of internal organs. This gene varies slightly from species to species but its overall functions seem to be similar.

Human embryonic development or human embryogenesis is the development and formation of the human embryo. It is characterised by the processes of cell division and cellular differentiation of the embryo that occurs during the early stages of development. In biological terms, the development of the human body entails growth from a one-celled zygote to an adult human being. Fertilization occurs when the sperm cell successfully enters and fuses with an egg cell (ovum). The genetic material of the sperm and egg then combine to form the single cell zygote and the germinal stage of development commences. Embryonic development in the human, covers the first eight weeks of development; at the beginning of the ninth week the embryo is termed a fetus. The eight weeks have 23 stages.

Nodal homolog is a secretory protein that in humans is encoded by the NODAL gene which is located on chromosome 10q22.1. It belongs to the transforming growth factor beta superfamily. Like many other members of this superfamily it is involved in cell differentiation in early embryogenesis, playing a key role in signal transfer from the primitive node, in the anterior primitive streak, to lateral plate mesoderm (LPM).

In avian gastrulation, Koller's sickle is a local thickening of cells at the posterior edge of the upper layer of the area pellucida called the epiblast. Koller's sickle is crucial for avian development, due to its critical role in inducing the differentiation of various avian body parts. Koller's sickle induces primitive streak and Hensen's node, which are major components of avian gastrulation. Avian gastrulation is a process by which developing cells in an avian embryo move relative to one another in order to form the three germ layers.
The development of the digestive system in the human embryo concerns the epithelium of the digestive system and the parenchyma of its derivatives, which originate from the endoderm. Connective tissue, muscular components, and peritoneal components originate in the mesoderm. Different regions of the gut tube such as the esophagus, stomach, duodenum, etc. are specified by a retinoic acid gradient that causes transcription factors unique to each region to be expressed. Differentiation of the gut and its derivatives depends upon reciprocal interactions between the gut endoderm and its surrounding mesoderm. Hox genes in the mesoderm are induced by a Hedgehog signaling pathway secreted by gut endoderm and regulate the craniocaudal organization of the gut and its derivatives. The gut system extends from the oropharyngeal membrane to the cloacal membrane and is divided into the foregut, midgut, and hindgut.














