
The mesoderm is the middle layer of the three germ layers that develops during gastrulation in the very early development of the embryo of most animals. The outer layer is the ectoderm, and the inner layer is the endoderm.

Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst, is reorganized into a two-layered or three-layered embryo known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.

The ectoderm is one of the three primary germ layers formed in early embryonic development. It is the outermost layer, and is superficial to the mesoderm and endoderm. It emerges and originates from the outer layer of germ cells. The word ectoderm comes from the Greek ektos meaning "outside", and derma meaning "skin".

Endoderm is the innermost of the three primary germ layers in the very early embryo. The other two layers are the ectoderm and mesoderm. Cells migrating inward along the archenteron form the inner layer of the gastrula, which develops into the endoderm.

The blastocyst is a structure formed in the early embryonic development of mammals. It possesses an inner cell mass (ICM) also known as the embryoblast which subsequently forms the embryo, and an outer layer of trophoblast cells called the trophectoderm. This layer surrounds the inner cell mass and a fluid-filled cavity known as the blastocoel. In the late blastocyst, the trophectoderm is known as the trophoblast. The trophoblast gives rise to the chorion and amnion, the two fetal membranes that surround the embryo. The placenta derives from the embryonic chorion and the underlying uterine tissue of the mother.
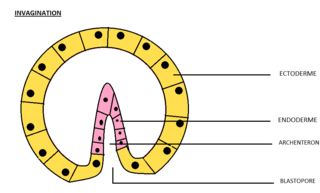
Invagination is the process of a surface folding in on itself to form a cavity, pouch or tube. In developmental biology, invagination is a mechanism that takes place during gastrulation. This mechanism or cell movement happens mostly in the vegetal pole. Invagination consists of the folding of an area of the exterior sheet of cells towards the inside of the blastula. In each organism, the complexity will be different depending on the number of cells. Invagination can be referenced as one of the steps of the establishment of the body plan. The term, originally used in embryology, has been adopted in other disciplines as well.

The blastocoel, also spelled blastocoele and blastocele, and also called cleavage cavity, or segmentation cavity is a fluid-filled or yolk-filled cavity that forms in the blastula during very early embryonic development. At this stage in mammals the blastula develops into the blastocyst containing an inner cell mass, and outer trophectoderm.
A germ layer is a primary layer of cells that forms during embryonic development. The three germ layers in vertebrates are particularly pronounced; however, all eumetazoans produce two or three primary germ layers. Some animals, like cnidarians, produce two germ layers making them diploblastic. Other animals such as bilaterians produce a third layer between these two layers, making them triploblastic. Germ layers eventually give rise to all of an animal's tissues and organs through the process of organogenesis.

In developmental biology, animal embryonic development, also known as animal embryogenesis, is the developmental stage of an animal embryo. Embryonic development starts with the fertilization of an egg cell (ovum) by a sperm cell, (spermatozoon). Once fertilized, the ovum becomes a single diploid cell known as a zygote. The zygote undergoes mitotic divisions with no significant growth and cellular differentiation, leading to development of a multicellular embryo after passing through an organizational checkpoint during mid-embryogenesis. In mammals, the term refers chiefly to the early stages of prenatal development, whereas the terms fetus and fetal development describe later stages.
The primitive node is the organizer for gastrulation in most amniote embryos. In birds it is known as Hensen's node, and in amphibians it is known as the Spemann-Mangold organizer. It is induced by the Nieuwkoop center in amphibians, or by the posterior marginal zone in amniotes including birds.

The primitive streak is a structure that forms in the early embryo in amniotes. In amphibians, the equivalent structure is the blastopore. During early embryonic development, the embryonic disc becomes oval shaped, and then pear-shaped with the broad end towards the anterior, and the narrower region projected to the posterior. The primitive streak forms a longitudinal midline structure in the narrower posterior (caudal) region of the developing embryo on its dorsal side. At first formation, the primitive streak extends for half the length of the embryo. In the human embryo, this appears by stage 6, about 17 days.
In embryology, Carnegie stages are a standardized system of 23 stages used to provide a unified developmental chronology of the vertebrate embryo.

The inner cell mass (ICM) or embryoblast is a structure in the early development of an embryo. It is the mass of cells inside the blastocyst that will eventually give rise to the definitive structures of the fetus. The inner cell mass forms in the earliest stages of embryonic development, before implantation into the endometrium of the uterus. The ICM is entirely surrounded by the single layer of trophoblast cells of the trophectoderm.

The bilaminar embryonic disc, bilaminar blastoderm or embryonic disc is the distinct two-layered structure of cells formed in an embryo. In the development of the human embryo this takes place by day eight. It is formed when the inner cell mass, also known as the embryoblast, forms a bilaminar disc of two layers, an upper layer called the epiblast and a lower layer called the hypoblast, which will eventually form into fetus. These two layers of cells are stretched between two fluid-filled cavities at either end: the primitive yolk sac and the amniotic sac.

A laminar organization describes the way certain tissues, such as bone membrane, skin, or brain tissues, are arranged in layers.

Human embryonic development or human embryogenesis is the development and formation of the human embryo. It is characterised by the processes of cell division and cellular differentiation of the embryo that occurs during the early stages of development. In biological terms, the development of the human body entails growth from a one-celled zygote to an adult human being. Fertilization occurs when the sperm cell successfully enters and fuses with an egg cell (ovum). The genetic material of the sperm and egg then combine to form the single cell zygote and the germinal stage of development commences. Embryonic development in the human, covers the first eight weeks of development; at the beginning of the ninth week the embryo is termed a fetus. The eight weeks have 23 stages.

In amniote embryology, the hypoblast is one of two distinct layers arising from the inner cell mass in the mammalian blastocyst, or from the blastodisc in reptiles and birds. The hypoblast gives rise to the yolk sac, which in turn gives rise to the chorion.

In avian gastrulation, Koller's sickle is a local thickening of cells at the posterior edge of the upper layer of the area pellucida called the epiblast. Koller's sickle is crucial for avian development, due to its critical role in inducing the differentiation of various avian body parts. Koller's sickle induces primitive streak and Hensen's node, which are major components of avian gastrulation. Avian gastrulation is a process by which developing cells in an avian embryo move relative to one another in order to form the three germ layers.
Embryogenesis in living creatures occurs in different ways depending on class and species. Organisms which are independent of a water habitat exhibit unique features during embryonic development. Amphibians are remnants of the first vertebrates which adapted the ability to survive in a mixed environment containing both water and dry land
This glossary of developmental biology is a list of definitions of terms and concepts commonly used in the study of developmental biology and related disciplines in biology, including embryology and reproductive biology, primarily as they pertain to vertebrate animals and particularly to humans and other mammals. The developmental biology of invertebrates, plants, fungi, and other organisms is treated in other articles; e.g terms relating to the reproduction and development of insects are listed in Glossary of entomology, and those relating to plants are listed in Glossary of botany.














