G protein-activated inward rectifier potassium channel 4(GIRK-4) is a protein that in humans is encoded by the KCNJ5 gene and is a type of G protein-gated ion channel. [5] [6]
G protein-activated inward rectifier potassium channel 4(GIRK-4) is a protein that in humans is encoded by the KCNJ5 gene and is a type of G protein-gated ion channel. [5] [6]
Potassium channels are present in most mammalian cells, where they participate in a wide range of physiologic responses. The protein encoded by this gene is an integral membrane protein and inward-rectifier type potassium channel. The encoded protein, which has a greater tendency to allow potassium to flow into a cell rather than out of a cell, is controlled by G-proteins. It may associate with other G-protein-activated potassium channel subunits to form a heterotetrameric pore-forming complex. [6]
In humans KCNJ5 is mainly expressed in adrenal gland and pituitary, although it is also detected at low levels in pancreas, spleen, lung, heart and brain. [7] Consistent with this expression pattern, mutations in KCNJ5/Kir3.4 can cause familial hyperaldosteronism type III and a type of long QT syndrome. [8]
Potassium channels are the most widely distributed type of ion channel found in virtually all organisms. They form potassium-selective pores that span cell membranes. Potassium channels are found in most cell types and control a wide variety of cell functions.

G protein-gated ion channels are a family of transmembrane ion channels in neurons and atrial myocytes that are directly gated by G proteins.

The renal outer medullary potassium channel (ROMK) is an ATP-dependent potassium channel (Kir1.1) that transports potassium out of cells. It plays an important role in potassium recycling in the thick ascending limb (TAL) and potassium secretion in the cortical collecting duct (CCD) of the nephron. In humans, ROMK is encoded by the KCNJ1 gene. Multiple transcript variants encoding different isoforms have been found for this gene.
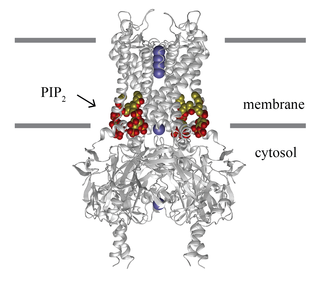
Inward-rectifier potassium channels (Kir, IRK) are a specific lipid-gated subset of potassium channels. To date, seven subfamilies have been identified in various mammalian cell types, plants, and bacteria. They are activated by phosphatidylinositol 4,5-bisphosphate (PIP2). The malfunction of the channels has been implicated in several diseases. IRK channels possess a pore domain, homologous to that of voltage-gated ion channels, and flanking transmembrane segments (TMSs). They may exist in the membrane as homo- or heterooligomers and each monomer possesses between 2 and 4 TMSs. In terms of function, these proteins transport potassium (K+), with a greater tendency for K+ uptake than K+ export. The process of inward-rectification was discovered by Denis Noble in cardiac muscle cells in 1960s and by Richard Adrian and Alan Hodgkin in 1970 in skeletal muscle cells.

The Kir2.1 inward-rectifier potassium channel is a lipid-gated ion channel encoded by the KCNJ2 gene.
An ATP-sensitive potassium channel is a type of potassium channel that is gated by intracellular nucleotides, ATP and ADP. ATP-sensitive potassium channels are composed of Kir6.x-type subunits and sulfonylurea receptor (SUR) subunits, along with additional components. KATP channels are found in the plasma membrane; however some may also be found on subcellular membranes. These latter classes of KATP channels can be classified as being either sarcolemmal ("sarcKATP"), mitochondrial ("mitoKATP"), or nuclear ("nucKATP").

Kir6.2 is a major subunit of the ATP-sensitive K+ channel, a lipid-gated inward-rectifier potassium ion channel. The gene encoding the channel is called KCNJ11 and mutations in this gene are associated with congenital hyperinsulinism.

G protein-activated inward rectifier potassium channel 2 is a protein that in humans is encoded by the KCNJ6 gene. Mutation in KCNJ6 gene has been proposed to be the cause of Keppen-Lubinsky Syndrome (KPLBS).

Potassium inwardly-rectifying channel, subfamily J, member 4, also known as KCNJ4 or Kir2.3, is a human gene.

Potassium inwardly-rectifying channel, subfamily J, member 8, also known as KCNJ8, is a human gene encoding the Kir6.1 protein. A mutation in KCNJ8 has been associated with cardiac arrest in the early repolarization syndrome.

ATP-sensitive inward rectifier potassium channel 12 is a lipid-gated ion channel that in humans is encoded by the KCNJ12 gene.

G protein-activated inward rectifier potassium channel 1(GIRK-1) is encoded in the human by the gene KCNJ3.

ATP-sensitive inward rectifier potassium channel 10 is a protein that in humans is encoded by the KCNJ10 gene.

Potassium inwardly-rectifying channel, subfamily J, member 15, also known as KCNJ15 is a human gene, which encodes the Kir4.2 protein.

Potassium inwardly-rectifying channel, subfamily J, member 16 (KCNJ16) is a human gene encoding the Kir5.1 protein.

Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-T2 is a protein that in humans is encoded by the GNGT2 gene.

G protein-activated inward rectifier potassium channel 3 is a protein that in humans is encoded by the KCNJ9 gene.

Potassium inwardly-rectifying channel, subfamily J, member 13 (KCNJ13) is a human gene encoding the Kir7.1 protein.
The G protein-coupled inwardly-rectifying potassium channels (GIRKs) are a family of lipid-gated inward-rectifier potassium ion channels which are activated (opened) by the signaling lipid PIP2 and a signal transduction cascade starting with ligand-stimulated G protein-coupled receptors (GPCRs). GPCRs in turn release activated G-protein βγ- subunits (Gβγ) from inactive heterotrimeric G protein complexes (Gαβγ). Finally, the Gβγ dimeric protein interacts with GIRK channels to open them so that they become permeable to potassium ions, resulting in hyperpolarization of the cell membrane. G protein-coupled inwardly-rectifying potassium channels are a type of G protein-gated ion channels because of this direct interaction of G protein subunits with GIRK channels. The activation likely works by increasing the affinity of the channel for PIP2. In high concentration PIP2 activates the channel absent G-protein, but G-protein does not activate the channel absent PIP2.
AsKC11 is a toxin found in the venom of the sea anemone, Anemonia sulcata. This toxin is part of the Kunitz peptide family and has been shown to be an activator of G protein-coupled inwardly-rectifying potassium (GIRK) channels 1/2, involved in the regulation of cellular excitability.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.